Loading...
225 view(s)
- Advances in Prenatal Diagnostics Set the Stage for Prenatal Therapies
- In Utero Delivery of Erythropoietin (EPO) Via mRNA Has Now Been Demonstrated
- Screening Novel Lipid Nanoparticle Carriers Was Necessary
Advances in DNA sequencing technology and
prenatal diagnostics, including the ability to detect
cell-free fetal DNA in maternal circulation, have enabled the diagnosis of many genetic diseases before birth. Although postnatal therapy by protein replacement and/or gene editing after birth is promising for many diseases, the pathology of some diseases begins before birth and is irreversible, resulting in prenatal or perinatal death or long-term morbidity (see
Ferreira & Gahl).

Prenatal therapy allows for treatment either before onset or in the early stages of irreversible pathology, which significantly reduces disease morbidity and mortality, especially in conditions caused by protein deficiencies that can be approached by delivery of either the needed protein or an mRNA encoding it (see
Trepotec et al. and refs. cited therein). Unlike direct delivery of proteins, the use of endogenous machinery to produce the therapeutic protein following mRNA delivery allows for natural
posttranslational modifications to occur.
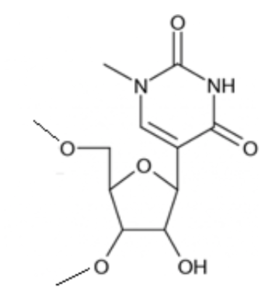 N1-methylpseudouridine (m1Ψ) moiety in modified mRNA. Taken from TriLink BioTechnologies.
N1-methylpseudouridine (m1Ψ) moiety in modified mRNA. Taken from TriLink BioTechnologies.
According to
Riley et al., whose 2021 publication is featured in this blog, the implementation of mRNA therapeutics for
in utero therapy required the development of novel mRNA delivery technologies. They therefore designed and screened lipid nanoparticle (LNP) platforms for mRNA delivery that could ultimately be used to treat monogenic fetal diseases with limited therapeutic options in the prenatal setting. This breakthrough research, summarized in the following sections, utilized
luciferase mRNA and
erythropoietin (EPO) mRNA, each modified with
N1-methylpseudouridine (m1Ψ) and 5’ capped using TriLink’s
CleanCap® technology, as detailed
elsewhere.
Overview
According to a recent article by
Schlich et al.,
ionizable LNPs are the most clinically advanced nano-delivery system for therapeutic nucleic acids. The researchers describe the “journey” of RNA-loaded LNPs across multiple intracellular barriers, from the extracellular space to the cytosol.
In silico molecular dynamics modeling,
in vitro high-resolution microscopy analyses, and
in vivo imaging data are systematically reviewed to distill out the regulating mechanisms underlying the endosomal escape of RNA.
As depicted here from Riley et al., ionizable lipids (synthesized by Michael addition chemistry), polyethylene glycol (PEG)-lipid, and 1,2-dioleoyl-
sn-glycero-3-phosphoethanolamine (DOPE) were combined into an ethanol phase, and a buffered solution of luciferase mRNA (m1Ψ, CleanCap®)
was diluted into an aqueous phase. Both phases were then mixed at controlled flow rates in microfluidic devices. After LNP formulation, LNPs were injected into individual mouse fetuses through the
vitelline vein, which directly delivers to
liver sinusoids in the fetus. After 4 or 24 hours, fetuses and tissues were extracted for luciferase-based imaging and further analysis.
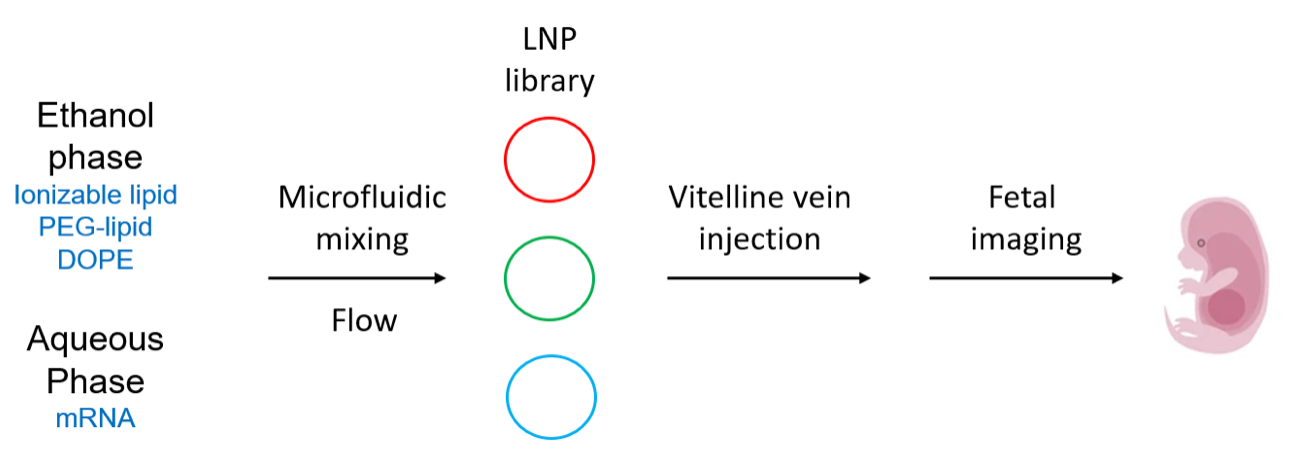 Overview of LNP formulation and fetal delivery. Drawn by Jerry Zon.
Overview of LNP formulation and fetal delivery. Drawn by Jerry Zon.
The use of ionizable lipids enables endosomal escape for efficient mRNA delivery to the cytosol, while LNPs can design and evaluate new ionizable polyamine-lipid structures within their formulations to optimize fetal delivery. The PEG, DOPE, and cholesterol excipients assist in LNP structural integrity, stability, and intracellular mRNA delivery. Following these optimizations, the
top LNP designs were used to deliver erythropoietin (EPO) mRNA to hepatocytes in mouse fetuses, which resulted in elevated levels of EPO protein in the fetal circulation and therefore a model for hepatocyte-mediated protein replacement therapy.
 3D illustration of a space-filling model of human erythropoietin (EPO), aka hematopoietin or hemopoietin, a cytokine secreted by kidneys that stimulates red blood cell production.
3D illustration of a space-filling model of human erythropoietin (EPO), aka hematopoietin or hemopoietin, a cytokine secreted by kidneys that stimulates red blood cell production.
A library of 14 LNPs was prepared using lipids synthesized by a
Michael-type addition reaction of a polyamine core with molar excesses of a terminal epoxide of a long-chain (C12, C14, and C16) alkyl compound. These reaction components are exemplified by the polyamine and C12 epoxide shown here. Together, they led to the top-performing LNP formulation (termed A-3) found by lipid/mRNA screening discussed below (see
Cheng et al. for synthesis of formally analogous lipids).
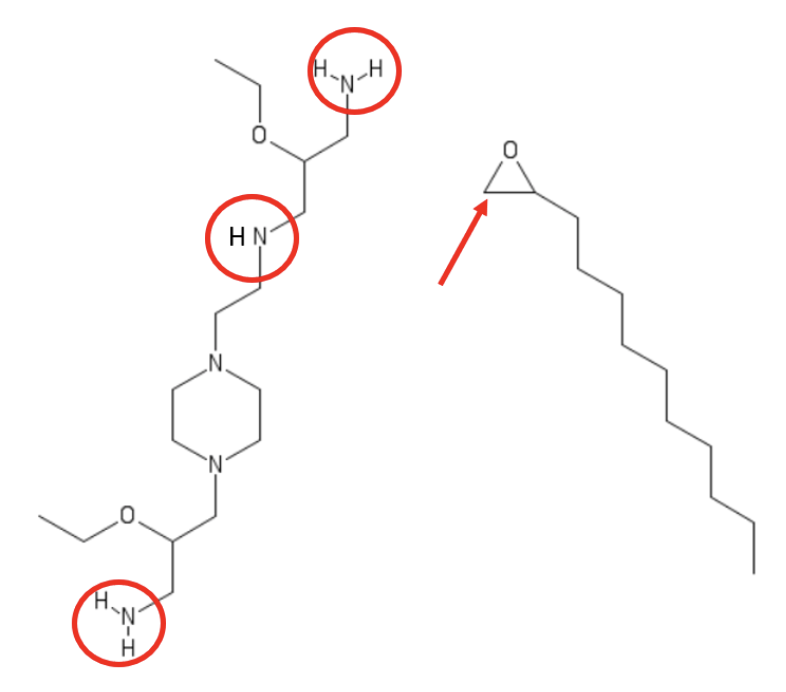 Exemplary polyamine and terminal epoxide alkyl (C12) Michael-type addition reactants that gave the top-performing formulation (termed A-3). Based on related publications by Park et al. and Cheng et al., the Zone assumes that each of red-circled amino moieties reacts with the epoxide by ring-opening at the less-hindered carbon center, indicated by the red arrow. Drawn by Jerry Zon.
Exemplary polyamine and terminal epoxide alkyl (C12) Michael-type addition reactants that gave the top-performing formulation (termed A-3). Based on related publications by Park et al. and Cheng et al., the Zone assumes that each of red-circled amino moieties reacts with the epoxide by ring-opening at the less-hindered carbon center, indicated by the red arrow. Drawn by Jerry Zon.
 2D illustration of chaotic mixing. Taken from commons.wikimedia.org and free to use.
2D illustration of chaotic mixing. Taken from commons.wikimedia.org and free to use.
Lipid/mRNA formulations were perfused through microfluidic devices designed with so-called “herringbone” features. These induce
chaotic mixing, as previously
reported. For an excellent video of herringbone microfluidic-based formation of LNPs, see this YouTube
link provided by Precision NanoSystems.
The hydrodynamic diameter measured for all LNP formulations ranged from 64 to 135 nm, and diameters were mostly (13-of-14) monodisperse in size distribution. Each LNP formulation was evaluated for its ability to encapsulate mRNA using RiboGreen assays, and all encapsulation efficiencies were high, ranging from 74 to 97.5%. Finally, LNPs were assessed for their p
Ka, the pH at which LNPs are 50% protonated. According to Riley et al., p
Ka values < 7.0 indicate that the LNPs will become protonated in endosomes, causing the lipids to fuse with the endosomal membrane for release of mRNA into the cytosol. On the other hand, p
Ka values of 6 to 7 are most commonly reported for
in vivo nucleic acid delivery. The measured p
Ka values for the LNP library were within the desired range for nucleic acid delivery, falling between 5.57 and 7.14.
LNPs Enable In Utero mRNA Delivery
Next, Riley et al. evaluated prenatal mRNA delivery via their LNPs using CleanCap® m1Ψ-modified luciferase mRNA, hereafter referred to simply as luciferase mRNA. This reporter enzyme uses an
in vivo imaging system (IVIS) and pregnant
BALB/c mice (dams) injected with luciferin (the required substrate, shown here), to enable direct visualization of transfection efficiency before administration of LNPs containing luciferase mRNA.
LNPs encapsulating luciferase mRNA were injected into gestational day (E) 16 mouse fetuses (minimum
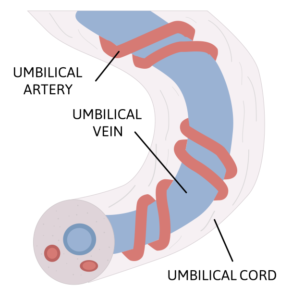
n = 3 fetuses) via the vitelline vein, and the injected dams and fetuses were assessed by IVIS 4 hours after injection. Each mouse fetus has its own gestational sac and vitelline vein, such that the vitelline vein injectate of one fetus does not cross over to additional fetuses. According to Riley et al., the vitelline vein drains directly into the fetal portal circulation, so
this mouse model represents injection of a mid-gestation human fetus umbilical vein, illustrated here.
 Albino BALB/c mice are among the most widely used inbred strains for animal experimentation. Taken commons.wikimedia.org and free to use.
Albino BALB/c mice are among the most widely used inbred strains for animal experimentation. Taken commons.wikimedia.org and free to use.
The sample size for each treatment varies, as the number of fetuses within the uterine horn of each dam varies. Thus, half of the fetuses in each dam were injected with an LNP formulation, while the other half were injected with phosphate-buffered saline (PBS) as an internal negative control for imaging. Analysis of pregnant dams following fetal injection revealed strong luciferase signal localized to the fetus for several LNP formulations, and no signal in any fetuses injected with PBS. In addition, according to Riley et al., no maternal tissues had luciferase signal, suggesting an absence of transplacental migration of the LNPs from fetus to dam or other fetuses in the uterine horn.
After imaging the dams, experimental and control fetuses were surgically removed to allow for more precise comparison of the efficiency of mRNA delivery between different LNPs. The fetuses were imaged individually by IVIS, and the normalized luminescent signal was subsequently quantified. In brief, four LNP formulations yielded greater mRNA delivery compared to others, indicating that the ionizable lipid within the LNPs strongly dictates their ability to deliver mRNA to fetuses. Importantly, no fetuses injected with naked (i.e., unformulated) mRNA yielded detectable luciferase signal.
LNPs Outperform Benchmark Lipid and Polymeric Delivery Systems
Riley et al. next compared their LNPs against the widely studied
in vivo nucleic acid delivery systems,
jetPEI (a linear polyethylenimine derivative) and
DLin-MC3-DMA (herein abbreviated MC3), for
in utero delivery. These were selected because “MC3 and jetPEI have been evaluated for nucleic acid delivery in clinical trials, thus making their comparison to the LNPs a critical benchmark for
in vivo mRNA delivery.” Furthermore, they add, the MC3 lipid was recently approved by the U.S. Food and Drug Administration (FDA) for clinical use for small interfering RNA (siRNA) delivery to treat polyneuropathy in patients with hereditary transthyretin-mediated amyloidosis (see
Zhang et al.).

Briefly, fetuses injected with luciferase mRNA formulated with jetPEI yielded only a modest increase in luminescence compared to naked luciferase mRNA 4 hours after injection, whereas the top-performing LNP formulation demonstrated a 75-fold increase in luminescence compared to jetPEI. Furthermore, this lead LNP formulation gave approximately 4-fold greater luminescence than an LNP prepared with MC3. Collectively, say Riley et al., these results indicated that
“LNPs developed in this study induced greater in utero protein expression in fetal livers than benchmark lipid and polymeric delivery systems currently used for nucleic acid delivery.”
LNPs Enable Delivery of mRNA in a Model for Protein Replacement Therapy
 3D illustration of a model of green fluorescent protein.
3D illustration of a model of green fluorescent protein.
EGFP mRNA expresses an enhanced version of the green fluorescent protein (GFP) originally isolated from the jellyfish,
Aequorea victoria. EGFP is a commonly used direct detection reporter in mammalian cell culture or
in vivo, and it is available from TriLink as
CleanCap® EGFP mRNA.
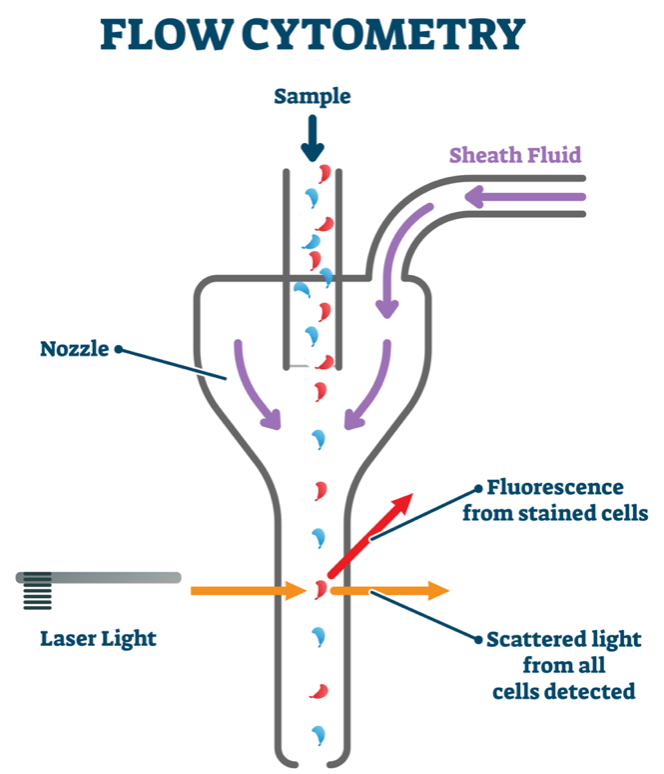
EGFP mRNA was encapsulated within an LNP comprised of the top-performing lipid formulation (termed LNP A-3/EGFP) derived from the polyamine/epoxide components shown above. LNP a-3/EGFP was delivered via the vitelline vein to E16 fetuses, and EGFP expression was assessed using fluorescent stereomicroscopy and flow cytometry 24 hours after injection. Similar to luciferase expression following injection of the corresponding A-3 LNPs loaded with luciferase mRNA, GFP was expressed in the fetal livers. Furthermore, treatment with LNP A-3/EGFP resulted in stronger GFP fluorescence in the liver at 24 hours after injection. For flow cytometry, fetal livers were processed into a single-cell suspension and stained for CD45, a marker for hematopoietic cells. The CD45
− population was used to determine the population of EGFP-expressing cells from the liver tissue, which revealed that LNP A-3 and B-4 yielded 1.1 % GFP
+ cells in the liver, consistent with the corresponding luciferase results.
Next, to demonstrate the
therapeutic potential of our LNPs, Riley et al. assessed prenatal delivery of
human EPO mRNA in LNP A-3 (LNP A-3/EPO). Successful liver delivery with LNP A-3/EPO would result in hepatic production of EPO protein, which is then secreted into the circulation. This model, they noted, is relevant to a number of enzyme deficiency disorders, such as the lysosomal storage diseases. These diseases cause irreversible damage before birth, which is why hepatic production and secretion of the deficient enzyme is being pursued as a viable therapy.
 Loading an ELISA plate to measure optical density (OD) with microplate reader.
Loading an ELISA plate to measure optical density (OD) with microplate reader.
LNP A-3/EPO was injected through the vitelline vein into E16 fetuses at two different doses (190 ng or 760 ng EPO mRNA), and fetal livers were assessed by enzyme-linked immunosorbent assays (ELISAs) for human EPO protein at 4 and 24 hours after injection. These were then compared to injection with PBS control, which did not show detectable human EPO. There was a clear dose-dependent response in EPO production following treatment with LNP A-3/EPO at both time points, specifically, 5-fold more EPO for the higher vs. low dose at 4 hours. Furthermore, fetal livers collected 24 hours after injection had lower EPO content compared to those collected 4 hours after injection. This was said to reflect the expected transient expression of the delivered EPO mRNA, and suggests that further studies of repeated injections of LNP-delivered mRNA are needed.
According to Riley et al., these results confirmed
“that these LNP platforms are robust and have the ability to deliver several types of mRNA, including those that model protein replacement therapies to treat prenatal disease.” Readers interested in additional details regarding the safety of such therapy can consult the original publication for various lines of experimental evidence demonstrating that this LNP/mRNA delivery did not induce fetal loss and resulted in minimal fetal immunotoxicity or liver damage.
Discussion
Based on the findings briefly summarized above—and many more not covered here—Riley et al. provide a lengthy discussion that should be read in full, as only some selected extracts are given here.
In the age of genomic medicine, a large number of clinically relevant monogenic diseases can be now be
diagnosed prenatally. Even though there are more than 100 postnatal enzyme and protein replacement therapies, these are limited by cost, adverse effects including hypersensitivity and immune response, limitations in reaching the desired organs, and an inability to reverse or rescue already damaged tissue. Gene therapy, specifically mRNA therapeutics, is rapidly gaining traction as a method of inducing protein or enzyme expression
in vivo, with multiple studies demonstrating a longer-term expression of the substrates compared to expression seen in conventional replacement therapy.
 3D illustration of a human fetus with internal organs.
3D illustration of a human fetus with internal organs.
However, note Riley et al., research involving prenatal mRNA delivery is in a nascent stage of development,
yet has multiple advantages compared to postnatal therapy. These include a larger population of progenitor cells that can be treated and expanded, reduced immunogenicity due to the immature fetal immune system, and most importantly, the ability to deliver therapeutics early or before the onset of phenotypic abnormalities.
Furthermore, prenatal therapy can enable maximization of injected dosage per weight to reduce therapy and manufacturing costs, as a human fetus at 16 weeks’ gestation weighs approximately 100 g, compared to 4000 g at birth. This translates to a 40-fold lower required dosage compared to postnatal therapy when administered at 16 weeks’ gestation.
 3D illustration of a liposome.
3D illustration of a liposome.
Thus, LNP-mediated delivery of mRNA has the potential to benefit the numerous fetal diseases that can be treated following prenatal diagnosis. In this study, the focus revolved around the investigation of ionizable lipid structures within LNPs specifically for mRNA delivery to fetuses, as these materials enable a substantial amount of compositional flexibility. For example, the polyamine cores are easily interchangeable with epoxides of different lengths to develop diverse libraries of materials. Furthermore, the excipients used to make LNPs, as well as the molar ratios, confer additional design flexibility to LNP composition, all of which affect delivery and transfection.
A notable finding was that the lead ionizable LNPs outperformed both jetPEI and LNPs comprised of the DLin-MC3-DMA lipid in terms of mRNA delivery and safety. According to Riley et al., “[t>
he ability of our top LNPs to outperform the commercially available and FDA-approved DLin-MC3-DMA lipid for mRNA delivery to fetuses is a key aspect of the innovation in this work.”
 Normal red blood cells seen by a microscope.
Normal red blood cells seen by a microscope.
Furthermore, they say, “EPO represents a potentially therapeutic and disease-relevant mRNA.” For example, fetal anemia is a serious condition that can arise from multiple etiologies, including alloimmunization, infection, genetic disorders such as lysosomal storage diseases and inherited anemias, and other structural abnormalities (fetal/placental tumors, vascular malformations, etc.). LNP delivery of EPO mRNA for the stimulation of endogenous red blood cell (RBC) production may provide an alternative treatment to multiple invasive fetal blood transfusions for select fetal anemias, including those of infectious etiology.
Concluding Comments
This featured work by Riley et al. and other mRNA-delivery studies covered in
previous blogs in the Zone collectively attest to the remarkable progress facilitated by what is now the relatively streamlined synthesis of 5’-capped, base-modified mRNA by improved
in vitro transcription (IVT). Notable in this regard are the merits of TriLink’s Cap 1 mRNA synthesis with co-transcriptional
CleanCap® Analog, detailed elsewhere by
McCaffrey and collaborators and by
Henderson et al.
The future for mRNA-based therapies is indeed bright.
What do you think?
Your comments are welcomed, as usual.
Please feel free to share this blog with your colleagues or on social media.


 N1-methylpseudouridine (m1Ψ) moiety in modified mRNA. Taken from TriLink BioTechnologies.
N1-methylpseudouridine (m1Ψ) moiety in modified mRNA. Taken from TriLink BioTechnologies. Overview of LNP formulation and fetal delivery. Drawn by Jerry Zon.
Overview of LNP formulation and fetal delivery. Drawn by Jerry Zon. 3D illustration of a space-filling model of human erythropoietin (EPO), aka hematopoietin or hemopoietin, a cytokine secreted by kidneys that stimulates red blood cell production.
3D illustration of a space-filling model of human erythropoietin (EPO), aka hematopoietin or hemopoietin, a cytokine secreted by kidneys that stimulates red blood cell production. Exemplary polyamine and terminal epoxide alkyl (C12) Michael-type addition reactants that gave the top-performing formulation (termed A-3). Based on related publications by Park et al. and Cheng et al., the Zone assumes that each of red-circled amino moieties reacts with the epoxide by ring-opening at the less-hindered carbon center, indicated by the red arrow. Drawn by Jerry Zon.
Exemplary polyamine and terminal epoxide alkyl (C12) Michael-type addition reactants that gave the top-performing formulation (termed A-3). Based on related publications by Park et al. and Cheng et al., the Zone assumes that each of red-circled amino moieties reacts with the epoxide by ring-opening at the less-hindered carbon center, indicated by the red arrow. Drawn by Jerry Zon. 2D illustration of chaotic mixing. Taken from commons.wikimedia.org and free to use.
2D illustration of chaotic mixing. Taken from commons.wikimedia.org and free to use.
 LNPs encapsulating luciferase mRNA were injected into gestational day (E) 16 mouse fetuses (minimum n = 3 fetuses) via the vitelline vein, and the injected dams and fetuses were assessed by IVIS 4 hours after injection. Each mouse fetus has its own gestational sac and vitelline vein, such that the vitelline vein injectate of one fetus does not cross over to additional fetuses. According to Riley et al., the vitelline vein drains directly into the fetal portal circulation, so this mouse model represents injection of a mid-gestation human fetus umbilical vein, illustrated here.
LNPs encapsulating luciferase mRNA were injected into gestational day (E) 16 mouse fetuses (minimum n = 3 fetuses) via the vitelline vein, and the injected dams and fetuses were assessed by IVIS 4 hours after injection. Each mouse fetus has its own gestational sac and vitelline vein, such that the vitelline vein injectate of one fetus does not cross over to additional fetuses. According to Riley et al., the vitelline vein drains directly into the fetal portal circulation, so this mouse model represents injection of a mid-gestation human fetus umbilical vein, illustrated here.
 Albino BALB/c mice are among the most widely used inbred strains for animal experimentation. Taken commons.wikimedia.org and free to use.
Albino BALB/c mice are among the most widely used inbred strains for animal experimentation. Taken commons.wikimedia.org and free to use. Briefly, fetuses injected with luciferase mRNA formulated with jetPEI yielded only a modest increase in luminescence compared to naked luciferase mRNA 4 hours after injection, whereas the top-performing LNP formulation demonstrated a 75-fold increase in luminescence compared to jetPEI. Furthermore, this lead LNP formulation gave approximately 4-fold greater luminescence than an LNP prepared with MC3. Collectively, say Riley et al., these results indicated that “LNPs developed in this study induced greater in utero protein expression in fetal livers than benchmark lipid and polymeric delivery systems currently used for nucleic acid delivery.”
LNPs Enable Delivery of mRNA in a Model for Protein Replacement Therapy
Briefly, fetuses injected with luciferase mRNA formulated with jetPEI yielded only a modest increase in luminescence compared to naked luciferase mRNA 4 hours after injection, whereas the top-performing LNP formulation demonstrated a 75-fold increase in luminescence compared to jetPEI. Furthermore, this lead LNP formulation gave approximately 4-fold greater luminescence than an LNP prepared with MC3. Collectively, say Riley et al., these results indicated that “LNPs developed in this study induced greater in utero protein expression in fetal livers than benchmark lipid and polymeric delivery systems currently used for nucleic acid delivery.”
LNPs Enable Delivery of mRNA in a Model for Protein Replacement Therapy
 3D illustration of a model of green fluorescent protein.
3D illustration of a model of green fluorescent protein. Next, to demonstrate the therapeutic potential of our LNPs, Riley et al. assessed prenatal delivery of human EPO mRNA in LNP A-3 (LNP A-3/EPO). Successful liver delivery with LNP A-3/EPO would result in hepatic production of EPO protein, which is then secreted into the circulation. This model, they noted, is relevant to a number of enzyme deficiency disorders, such as the lysosomal storage diseases. These diseases cause irreversible damage before birth, which is why hepatic production and secretion of the deficient enzyme is being pursued as a viable therapy.
Next, to demonstrate the therapeutic potential of our LNPs, Riley et al. assessed prenatal delivery of human EPO mRNA in LNP A-3 (LNP A-3/EPO). Successful liver delivery with LNP A-3/EPO would result in hepatic production of EPO protein, which is then secreted into the circulation. This model, they noted, is relevant to a number of enzyme deficiency disorders, such as the lysosomal storage diseases. These diseases cause irreversible damage before birth, which is why hepatic production and secretion of the deficient enzyme is being pursued as a viable therapy.
 Loading an ELISA plate to measure optical density (OD) with microplate reader.
Loading an ELISA plate to measure optical density (OD) with microplate reader. 3D illustration of a human fetus with internal organs.
3D illustration of a human fetus with internal organs. 3D illustration of a liposome.
3D illustration of a liposome. Normal red blood cells seen by a microscope.
Normal red blood cells seen by a microscope.







