Self-amplifying RNA (saRNA) vaccines encode the antigen of interest and replication elements from an alphavirus genome that lead to intracellular RNA amplification. Unlike conventional mRNA vaccines, which only encode the antigen of interest and are translated directly from the incoming mRNA molecules, saRNA can generate many copies of RNA in the target cell (Geall et al. 2012). Advantages of saRNA relative to mRNA may include longer duration of expression of the encoded antigen, potency at lower doses, amenity to co-expression of multiple antigens, and enhanced T cell responses. Additionally, the manufacturing productivity of a saRNA platform may be two orders of magnitude higher than that of an mRNA vaccine platform, as discussed in an expert review (Geall et al.2022).
These attractive possibilities underlie the increased interest in, and number of publications on, saRNA during the past five years as noted in an earlier Zone in with Zon blog. As we begin 2023, I selected a handful of publications on saRNA to highlight in this month’s blog post. They focus on four general topics:
- Antiviral saRNA Vaccines
- Anticancer saRNA Vaccines
- Formulating Carriers of saRNA Vaccines
- Thermostability of a Lyophilized saRNA Vaccine Formulation
The publications highlighted here use TriLink BioTechnologies products to support saRNA research and development. Most relevantly, TriLink’s proprietary CleanCap® AU Reagent, specifically designed for saRNAs, is discussed, and was recently launched in GMP grade to facilitate scaling from clinical to commercial development of saRNA-based products.
1. Antiviral saRNA Vaccines
The above-mentioned review (Geall et al. 2022) summarizes ten antiviral saRNA clinical trials initiated since 2015. Among these, eight are against SARS-CoV-2, one is for herpes simplex virus type 2, and one targets rabies. Most of the results from these trials are not yet published.
Interest in developing a saRNA vaccine for COVID-19 led to the production of a CleanCap® AU reagent-derived saRNA vaccine comprised of the genetic code for VEEV replicase and the spike protein of SARS-CoV-2 (Pollock et al. 2022). A lipid nanoparticle (LNP) formulation of this saRNA (LNP-nCoVsaRNA) proved to be safe in an initial clinical trial (COVAC1); however, neutralization of SARS-CoV-2 by participant sera was measurable in only 15% (6/39; 0.1 mg) to 48% (11/23; 5.0 mg) depending on dose level received, indicating that modifications to optimize humoral responses are required to realize a more effective vaccine (Pollock et al. 2022).
Additionally, a search of the ClinicalTrials.Gov database for currently recruiting studies of saRNA vaccines found links to details for three trials:
- Safety and immunogenicity evaluation of LNP-nCoVsaRNA in Uganda (NCT04934111);
- Safety, reactogenicity, and immunogenicity of a COVID-19 booster (NCT05370040) comprised of lyophilized nanostructured lipid carrier (NLC) saRNA (discussed in section 4 below)
- Safety, tolerability, and immunogenicity of saRNA vaccine preparations by Pfizer against influenza (NCT05227001).
It will be of interest to compare the findings from these ongoing Uganda (1.) and booster (2.) studies with the results of the above-mentioned COVAC-1 clinical trial. The influenza study (3.) is important in the context of seeking a more effective alternative for the currently administered seasonal flu vaccines.
2. Anticancer saRNA Vaccines
According to a recent review (Dailey et al. 2022), investigations of the potential efficacy of saRNA vaccines for cancer treatment have lagged those aimed at infectious diseases. Indications of the possible clinical use of saRNA cancer vaccines is supported by a preclinical study (Maine et al. 2021) that showed the ability of LNP-formulated saRNAs to elicit neoepitope-specific CD4+/CD8+ T cell responses and reduce tumor growth in a well-established colorectal cancer model in mice. Additionally, co-administration with immune checkpoint inhibitors synergized to further enhance these responses. Lastly, immunization of non-human primates was also able to elicit high-quality T cell responses.
More recently, a saRNA cancer vaccine targeting a neoepitope was discussed in TriLink’s October 2022 Research Spotlight (Palmer et al. 2022) wherein LNP-packaged personalized saRNAs, manufactured by TriLink, were studied in combination with the monoclonal antibodies nivolumab and ipilimumab in individuals enrolled in a Phase 1/2 clinical trial (NCT03639714).
Other researchers have explored the novel use of intratumor electroporation for enhanced delivery of saRNA cancer vaccines directly into tumor cells (Silva-Pilipich et al. 2022). The saRNA encoded interleukin-12 and was packaged in virus-like particles rather than in LNPs. Electroporation into subcutaneous hepatocellular carcinoma mouse tumors increased survival 3-fold compared to untreated control mice.
3. Formulating Carriers of saRNA Vaccines
The delivery of saRNA requires a platform to provide protection and enhance cellular uptake. The overarching principle is to use a lipid or polymer to compact the anionic RNA molecules, thus reducing the size of the resultant particle to facilitate cellular uptake. The two sections above provided examples of encapsulation of saRNA by LNPs.
In 2019, a Shattock-led team from Imperial College London (see section 1) published the first report comparing LNP formulations with cationic and ionizable lipids with saRNA either in the interior of a LNP (i.e., encapsulation) or on the exterior of a LNP (Figure 1) (Blakney et al. 2019).
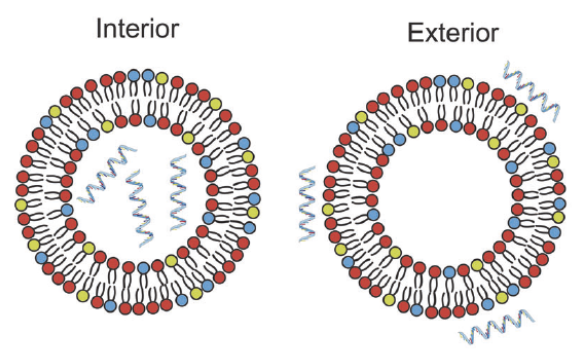
FIGURE 1. Schematic of saRNA formulated in the interior or on the exterior of LNPs. Taken from Blakney et al. 2019 and free to use under a Creative Commons Attribution 4.0 International License.
This publication showed that LNPs formulated with cationic lipids protected reporter saRNA from RNase degradation, even when the saRNA is adsorbed to the exterior surface. Furthermore, cationic LNPs delivered saRNA equivalently to particles formulated with saRNA encapsulated inside an ionizable LNP, both in vitro and in vivo. Finally, cationic and ionizable LNP formulations induced equivalent antibodies against saRNA-coded HIV-1 Env gp140 as a model antigen.
These teams subsequently published two noteworthy extensions of this work. First, an engineered bio-reducible cationic polymer for saRNA delivery led to enhanced cellular uptake and higher protein expression levels in vivo compared to commercially available polymers. When dosed mice were challenged with influenza, the saRNA conferred complete protection compared to untreated mice (Blakney et al. 2020). Second, saRNA formulations were systematically optimized by design-of-experiments using FDA-approved ionizable lipids in order to obtain maximum protein expression (Ly et al. 2022).
These reports collectively indicate that various types of lipids and other polymers can be used to formulate saRNA, which are typically larger than mRNA and may therefore require more optimization (Ly et al. 2022). As noted in the next section, formulated saRNA can also be lyophilized.
4. Thermostability of a Lyophilized saRNA Vaccine Formulation
The importance of developing and clinically testing lyophilized mRNA vaccines for enhanced stability, storage, and distribution, was discussed in an earlier Zone in with Zon blog.
For the first time, researchers have now extended lyophilization to a saRNA vaccine (Gerhardt et al. 2022). A lyophilized nanostructured lipid carrier (NLC) formulation of a saRNA (Figure 2) vaccine against Zika virus retained its biophysical properties and ability to induce protein expression in mice after at least 8 months of room temperature storage and at least 21 months at refrigerated temperatures in the lyophilized state. Similar stability was found for a lyophilized NLC-saRNA vaccine against COVID-19 (Voigt et al. 2022), suggesting possible general applicability.
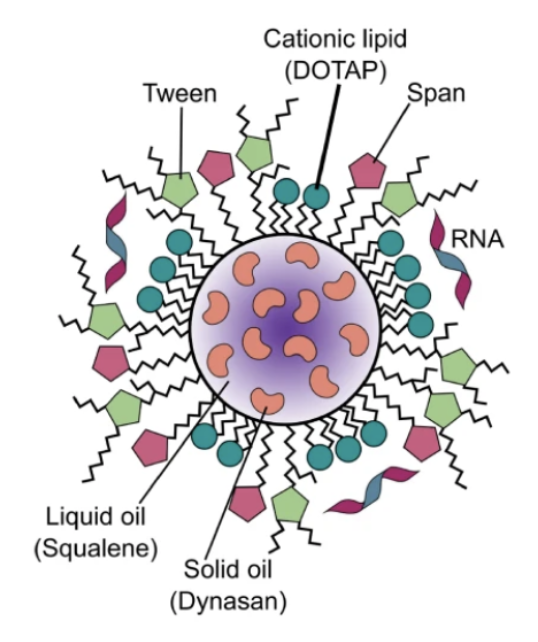
FIGURE 2. Schematic of saRNA formulated on the exterior of an NLC particle. Taken from Voigt et al. 2022 and free to use under a Creative Commons Attribution 4.0 International License.
In both cases, the novel NLC delivery system is comprised of an oil core of solid and liquid lipids surrounded by surfactants and a cationic lipid, as depicted in Figure 2. The saRNA is complexed electrostatically to the exterior of the NLC particle. This exterior surface attachment of the saRNA to the NLC particle was shown in vitro to prevent saRNA degradation by RNase.
These findings with an NLC represent a significant advance for improved non-frozen storage of saRNA, and it will be of interest to learn whether similarly improved storage is possible using conventional LNPs.
Concluding Comments
Despite the possible advantages of saRNA therapeutics, the clinical utility of saRNA has yet to be firmly established. Hopefully, the above-mentioned clinical trials and preclinical studies will lead to a compelling demonstration of the clinical benefit of saRNA, as has already been shown for approved mRNA vaccines for COVID-19. Particular attention will be focused on the clinical has driven saRNA development to date. Obtaining convincing clinical data for this assumed potency advantage for saRNA will likely require several appropriately designed comparative trials.
Your comments are welcomed, as usual.
Please feel free to share this blog with your colleagues or on social media.







