
CleanCap Technology: Leading the way in mRNA™
CleanCap® technology is a proprietary, co-transcriptional 5’ capping solution that generates a natural Cap 1 structure. Proper mRNA capping is critical to the production of the most biologically active and least immunogenic mRNA. TriLink scientists developed CleanCap, the next generation of capping technology, as a solution to the low capping efficiencies (mCAP/ARCA) or high enzyme costs that are associated with traditional capping methods.
CleanCap is efficient, elicits high yields of capped mRNA, and provides the highest quality mRNA 5’ cap structure available today. CleanCap is available in a variety of structures with specific initiation sequences. The newest structure is CleanCap AU, optimized for self-amplifying mRNA vaccine development. Our robust suite of CleanCap capping strategies is available for endless application possibilities.
Download our comprehensive CleanCap brochure and CleanCap AU technical flyer
Clean Cap Brochure Download
CleanCap AU Technical Flyer Download
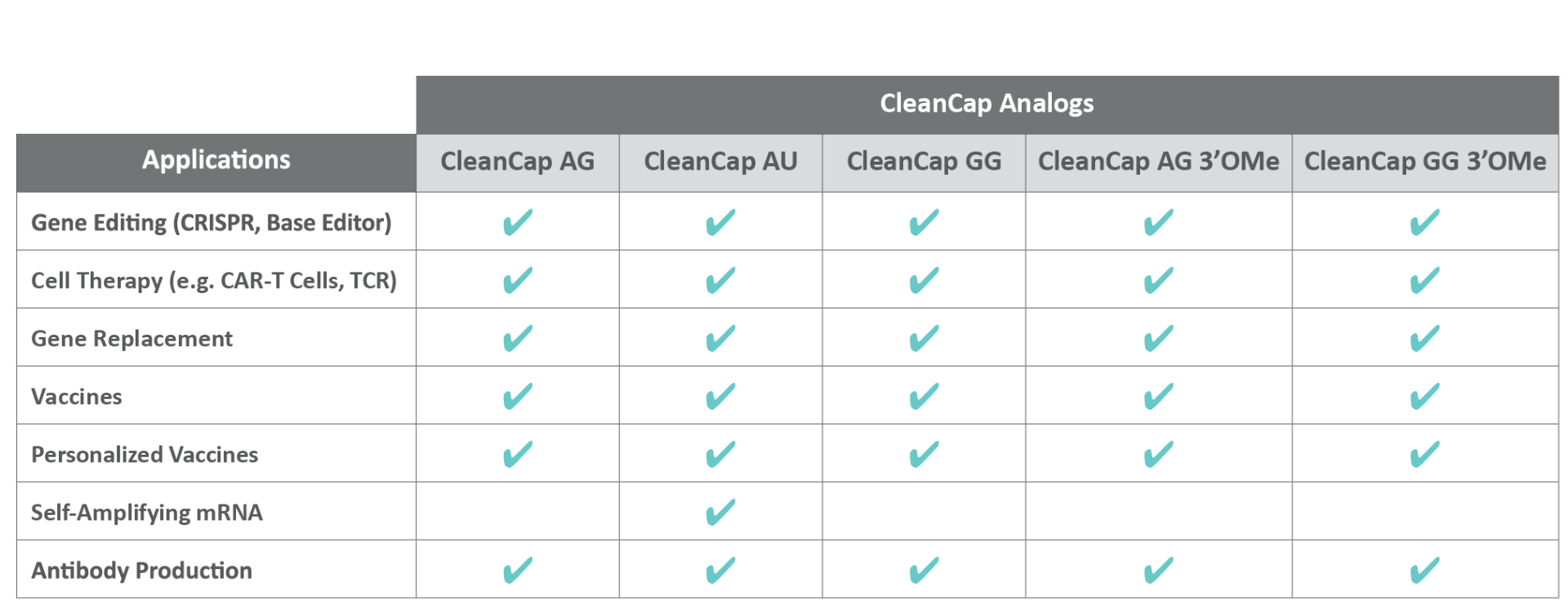
CleanCap is available as:
- GMP Custom mRNA Manufacturing
- Custom mRNA Manufacturing
- Capping Reagent — Discovery and GMPLink™ grades
- Stocked mRNA with CleanCap AG
- mRNA Manufacturing Including Plasmid Production
To find the CleanCap structure that is right for you, please fill out the form on this page and a member of our team will be in touch to assist you with technical support and product selection. The unparalleled value of CleanCap mRNA partnered with TriLink scientific and technical support expertise will drive your mRNA project to success.
Achieve optimal capping results
Successful development of mRNA therapeutics relies on reproducible, high-efficiency production of capped mRNA. CleanCap uses a robust new co-transcriptional capping process for the highest level of mRNA capping.
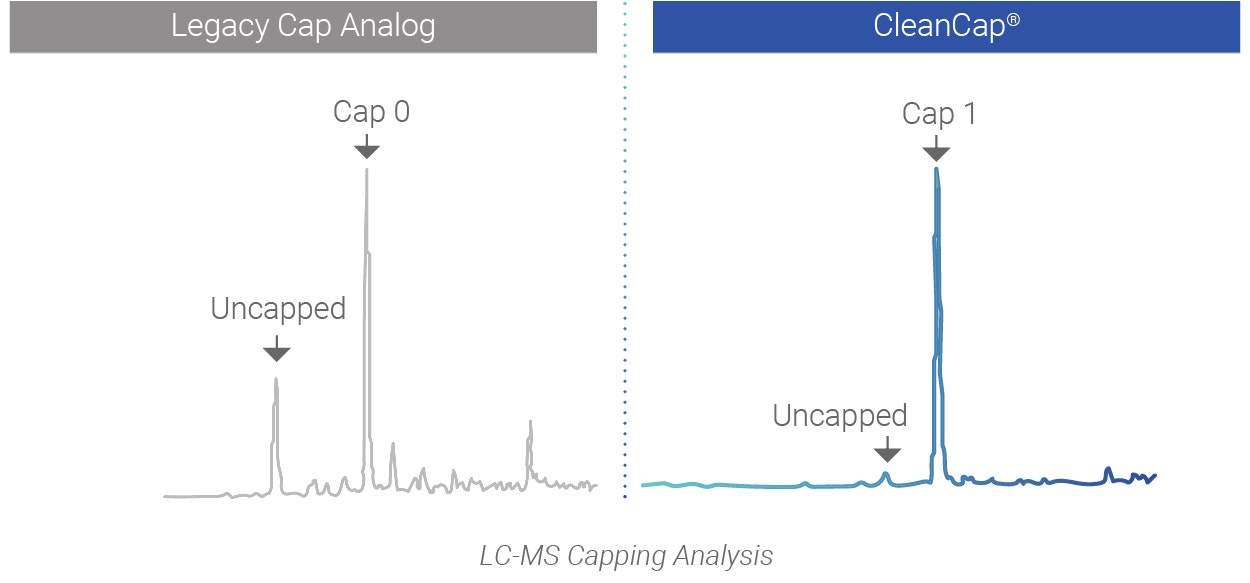
CleanCap demonstrates superior performance versus legacy co-transcriptional capping methods
The unique combination of expertise in synthetic organic chemistry and pioneering knowledge in mRNA at TriLink enabled us to invent a chemical solution for a biological problem.
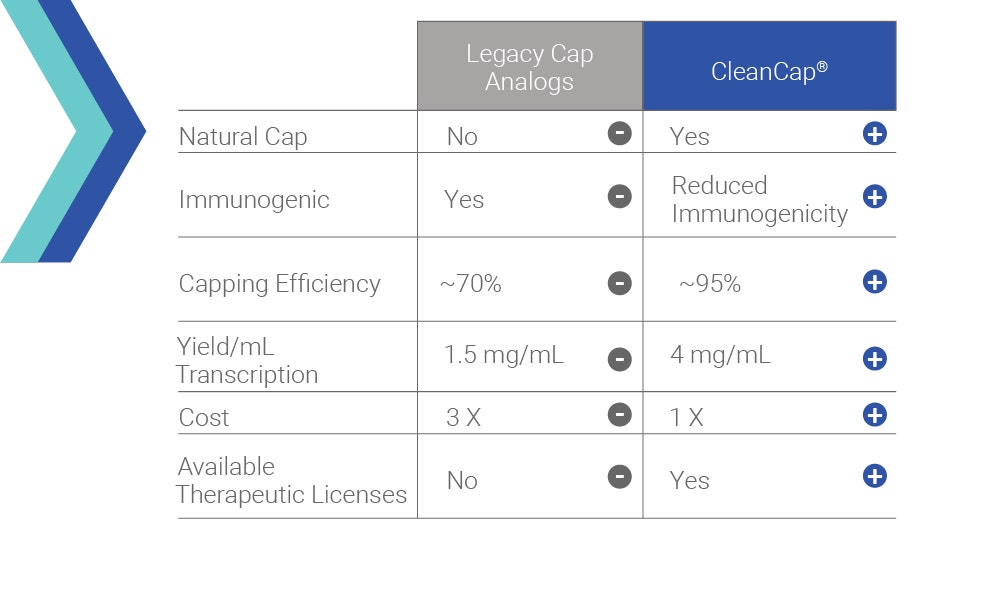
- High capping efficiencies (94%+) resulting in more active mRNA
- Yields a natural Cap 1 Product
- Cap 1 reduces activation of Pattern Recognition Receptors
- Dramatically increased activity in vivo in liver relative to Cap 0
- “One pot” co-transcriptional reaction to produce a Cap 1 structure vs multiple purifications steps required for enzymatic Cap 1
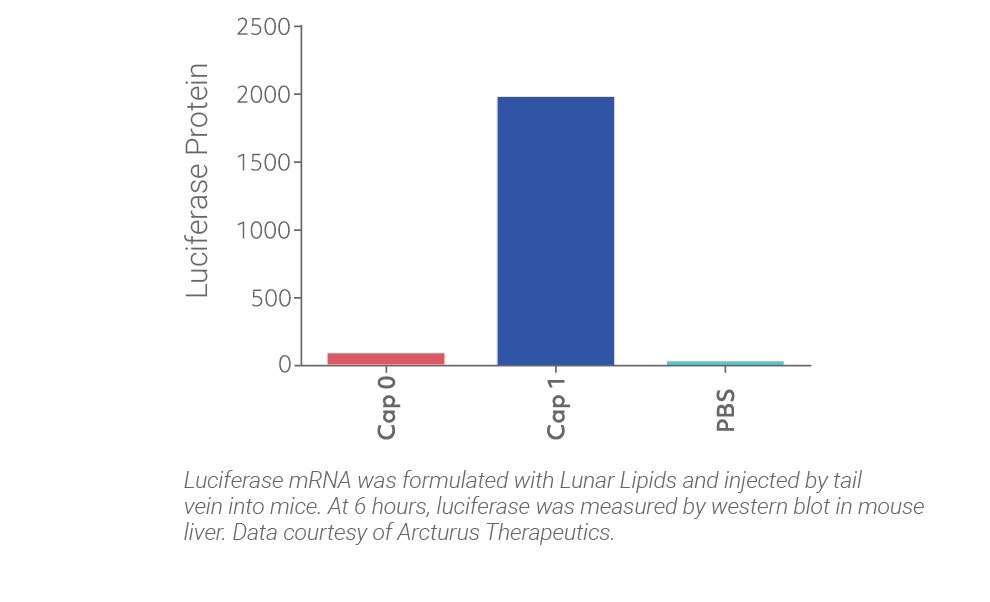
CleanCap gives superior activity in vivo by mimicking a natural cap
CleanCap results in a natural Cap 1 structure that reduces stimulation of the innate immune system of the host, resulting in unparalleled efficiency in vivo. Legacy co-transcriptional capping methods yield a Cap 0, an immunogenic cap structure that is poorly expressed in vivo.
The results speak for themselves: CleanCap, the next generation of cap analogs, provides the most active and least toxic mRNA for your in vivo applications.
Inquire about CleanCap Technologies
CleanCap Inquiry

Inquire About Incorporating CleanCap into Your Therapeutic GMP Manufacturing
CleanCap can improve your mRNA product and reduce your overall GMP cost.
Request a Consultation

