The mRNA Cap is the Capt’n of Your Vaccine Process
The effectiveness of an mRNA vaccine depends on the stability and expression of the injected mRNA construct. The 5’ 7-methylguanosine cap of the mRNA molecule is critical to its success, preventing cytoplasmic degradation, promoting ribosomal recruitment, and ensuring efficient translation. Constructs with incomplete caps or Cap 0 structures are at risk of degradation or immune-mediated silencing, making a Cap 1 structure the optimal choice for mRNA vaccine design.
CeanCap reagents offer a “one-pot” solution that reduces manufacturing time and eliminates the need for advanced skills and equipment to produce a Cap 1 structure, resulting in optimal performance without sacrificing safety, quality, or efficiency.
Choose the Right Analog for Your Application:
- CleanCap Reagent AG: produces a naturally occurring Cap 1 structure with efficiency and the lowest cost
- CleanCap Reagent AG 3’OMe: produces a modified Cap 1 structure with additional modifications to the m7G cap structure that improves protein translation
- CleanCap Reagent AU: produces a Cap 1 structure that is compatible with self-amplifying RNA alphavirus 5’ UTR sequences
Available in RUO and GMP grade so you can swiftly scale from clinical to commercial
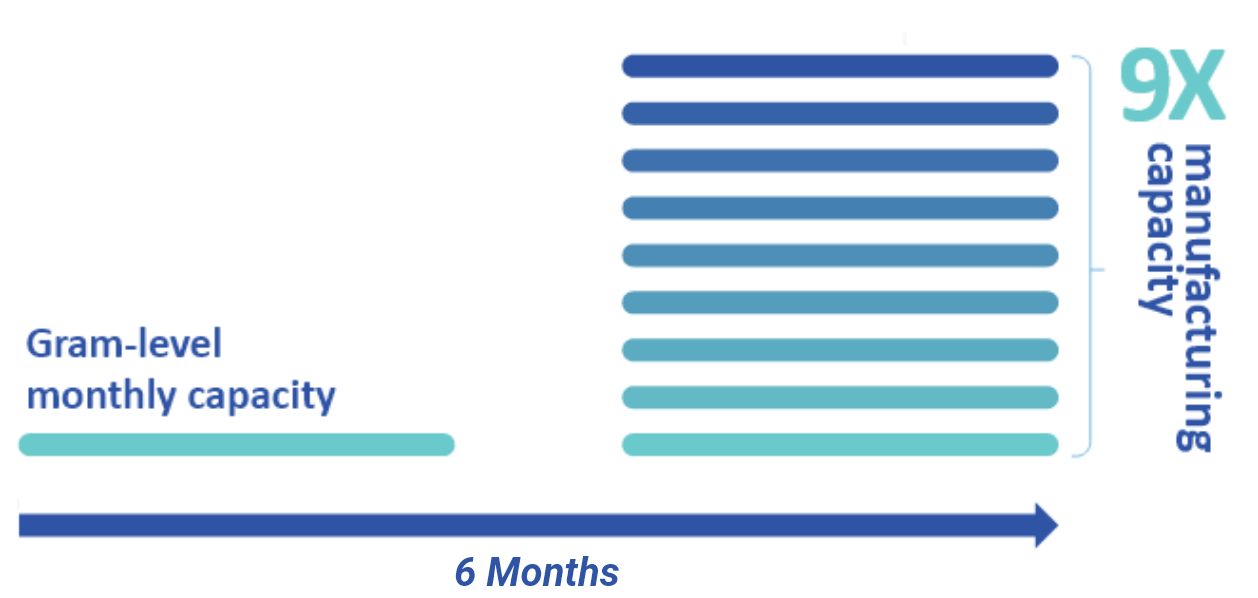
CleanCap Reagents Enable a Rapid Vaccine Response to a Global Health Crisis:
See how we ramped up production at unprecedented timelines and scales. CleanCap reagents are used in the leading COVID-19 vaccine approved/authorized for use in >140 countries. View case study >
Select Publications Using CleanCap Reagents in Vaccine Development
Cap 1 Messenger RNA Synthesis with Co-transcriptional CleanCap® Analog by In Vitro Transcription
This process in Current Protocols describes the co-transcriptional mRNA capping method using CleanCap AG reagent, which results in 5 mg/ml of IVT with 94% 5′-cap 1 structure.
Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine
This study, published in The Journal of New England Medicine, found that a two-dose regimen of the BNT162b2 vaccine, made using CleanCap reagents, conferred 95% protection against COVID-19 in humans.
A Dual-Antigen Self-Amplifying RNA SARS-CoV-2 Vaccine Induces Potent Humoral and Cellular Immune…
In this paper, published in Molecular Therapy, researchers demonstrated that the LNP-formulated dual-antigen self-amplifying RNA (saRNA) vaccine for SARS-CoV-2, made using CleanCap reagents, induced potent immune response.
mRNA Vaccination Induces Tick Resistance and Prevents Transmission of the Lyme Disease Agent
Sajid et al. proved that guinea pigs vaccinated with a 19ISP mRNA vaccine that used CleanCap reagents were protected against tick-borne illnesses, including Lyme disease.
Safety and Immunogenicity of a Self-Amplifying RNA Vaccine Against COVID-19: COVAC1, a Phase I…
In this phase I first-in-human trial published in The Lancet, Pollock et al. found that the lipid nanoparticle (LNP) saRNA COVID-19 vaccine made with CleanCap reagents was well-tolerated and caused no serious adverse reactions.
A phase 1, Randomized, Placebo-Controlled Study to Evaluate the Safety and Immunogenicity of an mRNA…
Aliprantis et al. evaluated an mRNA vaccine for Respiratory Syncytial Virus (RSV), made using CleanCap reagents, and found it was well-tolerated, caused no serious adverse events, and elicited an immune response.
Start Scaling Your Vaccine Faster Today
Talk to our team about what may be the best option for you. Fill out the form below to get started.


