Achieve optimal capping results.
Successful development of mRNA therapeutics relies on reproducible, high-efficiency production of capped mRNA. CleanCap uses a robust new co-transcriptional capping process for the highest level of mRNA capping.

Proper mRNA capping is critical to the production of the most biologically active and least immunogenic mRNA. TriLink scientists developed CleanCap, the next generation of capping technology, as a solution to the low capping efficiencies (mCAP/ARCA) or high enzyme costs that are associated with ARCA or enzymatic capping.
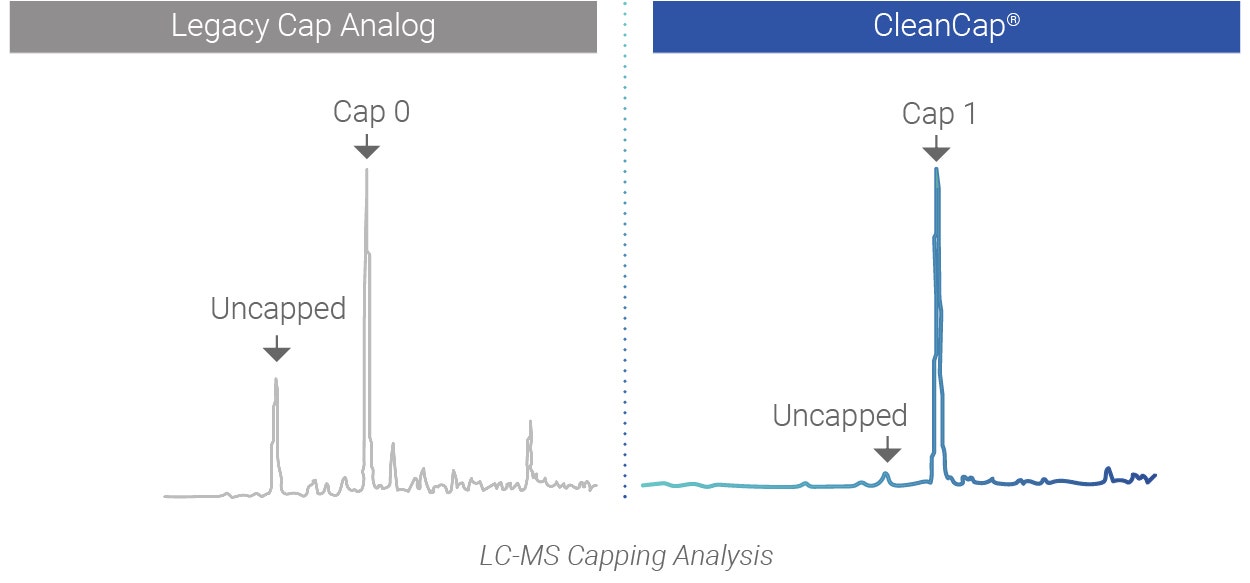
Successful development of mRNA therapeutics relies on reproducible, high-efficiency production of capped mRNA. CleanCap uses a robust new co-transcriptional capping process for the highest level of mRNA capping.
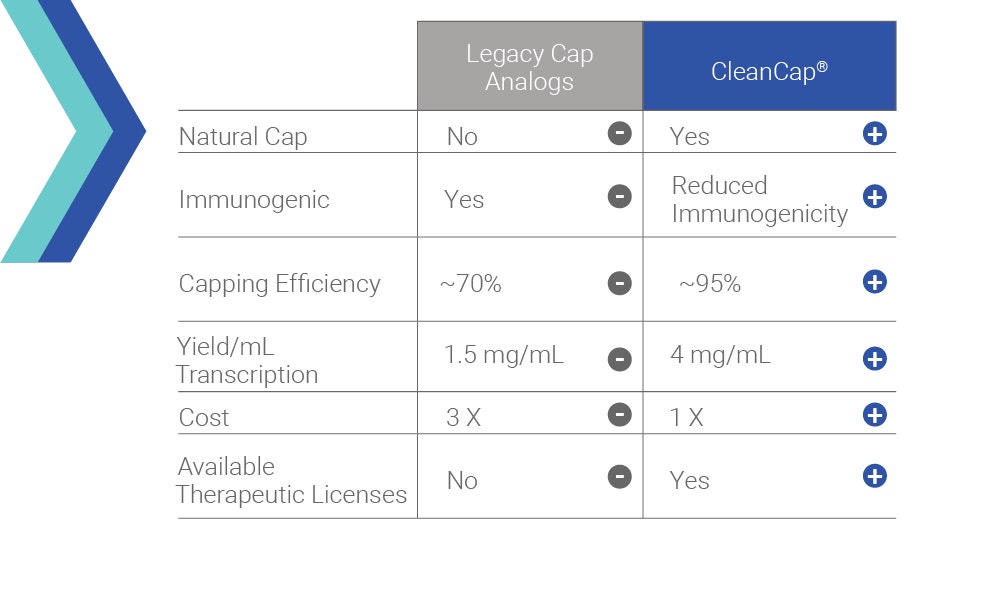
The unique combination of expertise in synthetic organic chemistry and pioneering knowledge in mRNA at TriLink enabled us to invent a chemical solution for a biological problem.
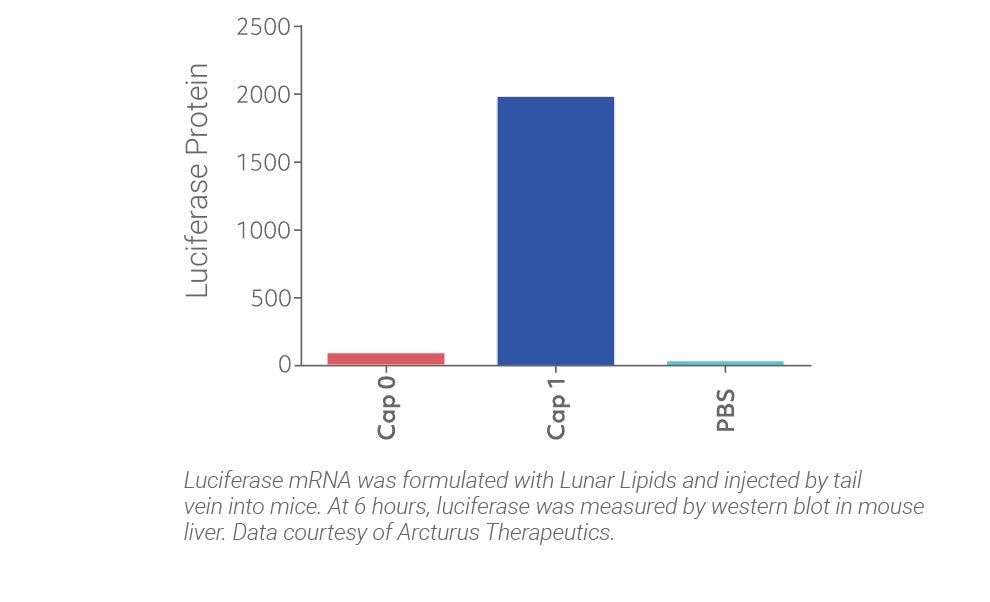
CleanCap results in a natural Cap 1 structure that reduces stimulation of the innate immune system of the host, resulting in unparalleled efficiency in vivo. Legacy co-transcriptional capping methods yield a Cap 0, an immunogenic cap structure that is poorly expressed in vivo.
The results speak for themselves: CleanCap, the next generation of cap analogs, provides the most active and least toxic mRNA for your in vivo applications.

CleanCap can improve your mRNA product and reduce your overall GMP cost.
Request a Consultation