
Closer to a Cure? Elucidating the Structure of the HIV Reverse Transcriptase Initiation Complex
July 2018
Last month, in a study published in Nature, a team of researchers from Stanford University unraveled the structural basis of the HIV reverse transcriptase initiation complex (RTIC), the discovery of which may have brought us closer to a cure for HIV.
Although the structure of each HIV component has previously been elucidated (see Figure 1), the structural basis of the initiation of transcription has remained elusive despite lasting efforts to uncover it.
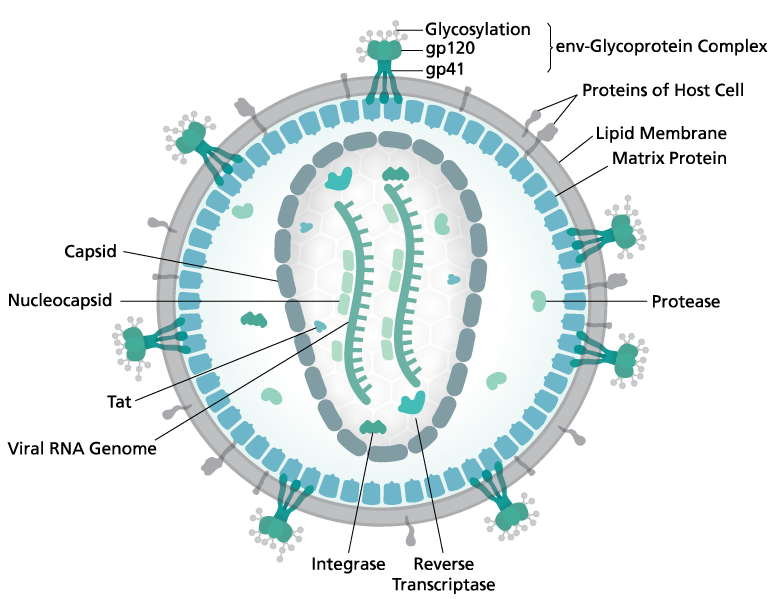
Understanding this process provides new opportunities for drug targeting, which could be essential for maintaining control of the rapidly mutating virus.
"A rich body of structural data on reverse transcriptase (RT), a heterodimer of p51 and p66 sub-units, has shown how its polymerase and RNase H domains interact with DNA–DNA and DNA–RNA duplexes in the absence and presence of antiviral drugs. Lacking, however, are structures that reflect initiation, showing how RT binds to a large bimolecular viral RNA(vRNA)-tRNALys complex," the authors explain.
Using a combination of cryo-electron microscopy, biochemical, and biophysical experiments, this group of researchers built an RTIC model. In this model, the RT is in an inactive conformation with open fingers and thumb, while the primer-template complex is turned away from the active site. The primer binding site (PBS) is located in the cleft of the RT.
The tRNA stacks and refolds on the PBS to form a helical structure, while the remaining vRNA creates two helical stems located above the RT active site. (See the publication for further description and an image of the RTIC structure.)
The researchers note that this model is consistent with previous reports describing how the initiation process is generally slower than elongation. During the initiation of reverse transcription, RT binds to the vRNA-tRNA Lys complex and then must traverse a highly-structured region, resulting in RT pausing at specific locations.
"As reverse transcription proceeds, structural rearrangements in vRNA and tRNA must occur to favor the transition to processive elongation. Thus, the initiation complex is likely to change progressively as initiation proceeds, and may be specifically vulnerable to inhibition by drugs," the authors explain.
Therefore, although this first step is monumental for the HIV field and the people living with HIV-1, further studies into the structural changes during these different states of initiation will be required in order to fully understand the RNA conformations that regulate HIV-1.
Featured product: 5-Amino-G-monophosphate (GMP), crosslinkable tRNALys3, custom synthetic oligonucleotides with sequences 5-GCGGGAGAUCAGGCAU(Am6)-cyanine5-3 and 5-biotin CUAUUCCCU-AUCCdC-3.
Have a question? Visit Ask An Expert.
© 2018 TriLink BioTechnologies. | All Rights Reserved

