Conquering Irritable Bowel Disease by Targeting Leukocytes
January 2019
As recently reported in Nature Communications, scientists from Tel Aviv University have used targeted expression of interleukin 10 (IL-10) mRNA in Ly6c+ inflammatory leukocytes to bring new hope to people suffering from irritable bowel disease (IBD).
This is the first in vivo delivery system targeting leukocytes that has resulted in selective and efficient mRNA-based protein expression.
IBD refers to a group of intestinal disorders, including Crohn’s disease and ulcerative colitis. These disorders are characterized by a complex and dysregulated immune response and are associated with an imbalance of the intestinal microbiota, resulting in chronic inflammation of the intestinal tract.
To combat this, the authors focused on increasing IL-10 expression, an important anti-inflammatory cytokine involved in balancing intestinal immunity. However, they also acknowledge that past clinical trials using recombinant IL-10 have failed to induce a robust therapeutic effect.
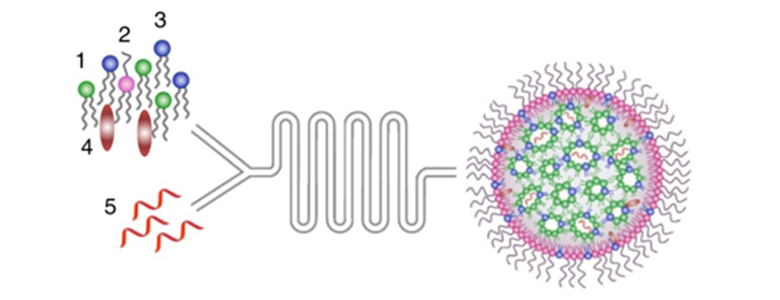
Figure 1: Schematic illustration of lipid nanoparticles (LNPs) preparation. A microfluidic based mixing of lipids, helper lipid DSPC (1), PEG-lipid (2), ionizable amino lipid DLin-MC3-DMA (3) and cholesterol (4), diluted in ethanol and modified mRNA (mmRNA)(5) diluted in acidic acetate buffer (pH 4) to construct LNPs encapsulated with mmRNA. Upon mixing in acidic condition, electrostatic interactions drive the formation of inverted micelles containing mmRNA molecules surrounded predominantly by ionizable lipid, which are then coated with PEG-lipids to form the LNPs. After LNP formation, the pH is raised to physiological pH, leading to LNPs with a biocompatible neutral charge. Subsequently, upon endocytosis to the acidic endosome, the amino group of the DLin-MC3-DMA becomes positively charged, which allows the release of mmRNA molecules to the cytosol. Figure courtesy of Veiga et al. 2018 (Figure 1a), published under the Creative Commons Attribution 4.0 International license.
"The main hurdles with recombinant IL-10 treatment were the short half-life of only a few hours and the high protein concentration needed for systemic administration, which resulted in increased toxicity," the authors explain.
To address these issues, the authors sought a temporal and spatial immunosuppressive effect in order to reduce the autoimmune response without affecting the body’s normal immune activity.
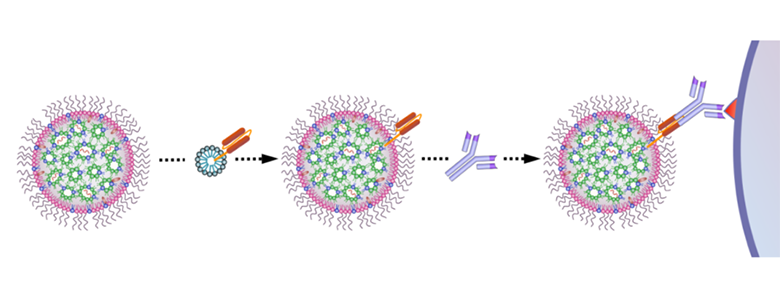
They did this by using a modular targeting platform called ASSET (Anchored Secondary scFv Enabling Targeting) to encapsulate IL-10 mRNA in lipid nanoparticles (LNPs) that target Ly6c+ inflammatory cells (see Figure 1). The ASSET platform works by coating the LNPs with monoclonal antibodies, while also allowing for switching between different antibodies (see Figure 2).
Figure 2: Schematic illustration of the introduction of targeting moiety to mmRNA loaded LNPs. LNPs are mixed with ASSET micelles and coated with Rat IgG2a primary mAbs, which promote selective binding of tLNPs to the target receptor. Figure courtesy of Veiga et al. 2018 (Figure 1f), published under the Creative Commons Attribution 4.0 International license.
Using a colitis model where mice are injected with dextran sodium sulfate, the system ultimately demonstrated selective protein expression of IL-10 in Ly6c+ leukocytes with a favorable biodistribution.
Selective IL-10 expression was shown to be effective in significantly lowering the amount of tumor necrosis factor alpha and IL-6 pro-inflammatory cytokines and reducing pathological signs of colitis, such as weight loss and erythema, which refer to a shortening of the inflamed colon or swelling and inflammatory infiltration of the colon, respectively.
Importantly, the therapy demonstrated a favorable safety profile without significant liver toxicity or measurable immunogenicity.
As with most novel therapies, the authors note some need for optimization, however the overall strategy appears promising for those who are suffering from IBD.
Featured product: CleanCap Firefly luciferase modified mRNA and Custom CleanCap IL-10 modified mRNA.
Have a question? Visit Ask An Expert.