
Smac’ing Down Tumor Cells with TriLink Cas9 mRNA
July 2017
A recent discovery reported in Molecular Cell demonstrated that inhibition of p38, an integral molecule of the tumor necrosis factor (TNF)-signaling cascade, improves smac-mimetic (SM)-based therapeutic approaches for cancer treatment, particularly in patients with acute myeloid leukemia.
SMs are a class of molecules that target the TNF-associated apoptotic pathway and induce cancer cell death. However, resistance is often acquired during the course of treatment, prompting research into combination therapies to improve and prolong the effectiveness of the treatment.
Although it was initially apparent that p38 inhibitors sensitized the cells to SMs and improved treatment outcomes, the mechanism of action was not understood, leading a group of international researchers to tease out these details in this paper.
TNF is an inflammatory cytokine that can induce cytokine production and drive cell survival or cell death through signaling cascades. Since p38 and its substrate MK2 are essential to TNF-induced inflammatory cytokine production, the group hypothesized that p38-MK2 arm of the cascade also controls TNF- and RIPK1-dependent SM-induced cell death (see Figure 1).
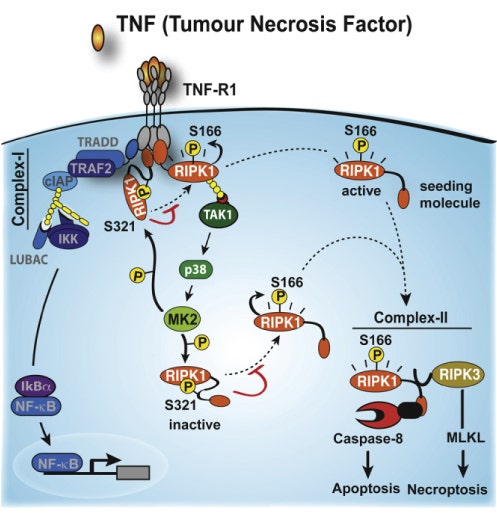
"Because SM kills cells by increasing the production of SM-induced TNF biosynthesis, and sensitizing cells to TNF-induced and RIPK1-mediated cell death, p38/MK2 might influence the sensitivity to TNF by influencing either or both of these processes," the authors explain.
Indeed, they reported that inhibition of p38 sensitizes cells independent of its role in TNF production. Specifically, they reported that the molecule downstream of p38, MK2, protects cells from TNF-induced cell death by inactivating RIPK through phosphorylation at site S321. Therefore, by inhibiting p38 and MK2, RIPK remains active and retains its ability to promote caspase activation and (tumor)cell death (see Figure 1).
Additionally, they found that MK2 ultimately acts by limiting the formation of complex II, which is essential for caspase activation and cell death. Interestingly, they also noted that it does not appear to impede NF-kB, a molecule downstream of complex I, that is important for the transcriptional induction of pro-inflammatory cytokines and pro-survival genes. This was surprising since defects in NF-kB are known to sensitize cells to TNF-induced cell death.
Using TriLinks Cas9 mRNA, the authors then created RIPK1 S321D phospho-mimetic knock-in mice. They were able to convincingly show in vivo that MK2-dependent phosphorylation of RIPK1 at site S321 protects cells from TNF-induced cell death, demonstrating a clear mechanism of action for p38 inhibition in tumor cells treated with Smac mimetics.
"It is particularly intriguing that p38a/MK2 inhibition increases both facets of SM activity [by production of TNF cytokines and increasing sensitivity to TNF-induced and RIPK1-mediated cell death], suggesting a deeper connection between TNF production and TNF-induced cell death than previously anticipated. While the details of this link remain unclear, these new insights provide a further rationale for exploring the combined treatment of p38/MK2i and SM against cancers clinically," conclude the authors.
Featured Products: Cas9 mRNA. TriLink has a number of options for your CRISPR/Cas9 needs, including modified Cas9 mRNA, Cas9 nickase mRNA, and guide strands. For custom projects, contact us at info@trilinkbiotech.com.
Have a question? Visit Ask An Expert.

