
Unraveling Cell Specific Muscular Disorders with TriLink’s Cas9
November 2017
Recently, researchers from University of Texas used the TriLink Cas9 mRNA to uncover a possible cause of one form of congenital myopathy.
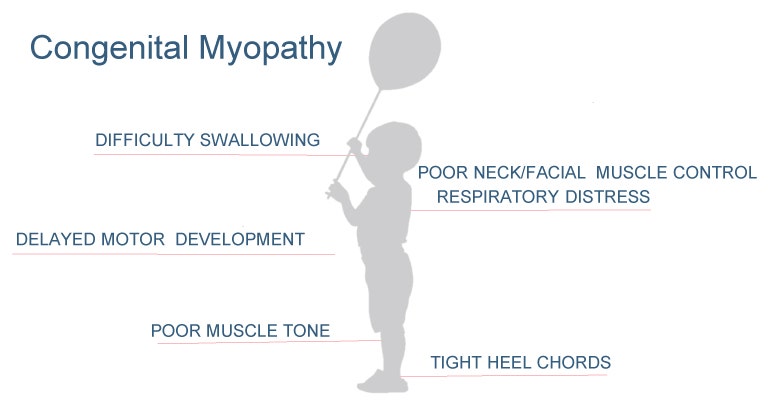
Congenital myopathy is a group of inherited muscle disorders that occurs in approximately 1 in 10,000 people. Symptoms are often identified at birth and causes muscle weakness, a delay in development, and in some cases may lead to death due to respiratory distress (see Figure 1).
"To date, there are no effective therapies for any of these disorders, and much remains to be learned about the underlying disease mechanisms," the researchers note.

The article, published last month in Journal of Clinical Investigation, used the TriLink Cas9 mRNA to knock out Kelch family protein Klhl31, in mice. The Kelch super family of proteins primarily affect protein stability, acting as substrate specific adapters that modulate ubiquination.
In previous studies, mice deficient of other select Kelch family proteins have shown characteristics of nemaline myopathy, which is characterized by protein aggregates within muscle fibers and results in muscle weakness that most severely affects the muscles of the face, neck, and limbs.
Mutations in Klhl40 have even been documented in humans with nemaline myopathy, suggesting an important role for this family of proteins in muscular disorders. However, the role of Klhl31, which is highly upregulated in skeletal muscle, had yet to be tested.
After creating knock-out mice using CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) technology, the researchers found that Klhl31 knockout mice were born healthy, but that they showed abnormal, postnatal skeletal development and reduced muscle integrity and function starting at postnatal Day 10. Further tests showed centronuclear myopathy (see Figure 2), sarcomeric degeneration, Z-disc streaming, and a dilated sarcotubular network.
To further understand the mechanisms involved, the researchers then used a proteomics approach to identify substrates of klhl31. Through this approach, they identified an upregulation of several proteins that have been linked to muscular dystrophies or congenital myopathies in humans, such as FlnC, Sgcb, Dnajb6, Bag3, Lamin A/C, Dag1, and Klhl4.
"We propose that Klhl31 is required to maintain normal protein turnover at the Z-disc and that, in the absence of Klhl31, a toxic accumulation of proteins manifests, resulting in destabilization of the Z-disc and subsequently the myofibril."
Although it is still unknown whether humans with congenital myopathy carry mutations in Klhl31, further studies will provide further insight to understanding the cellular mechanisms of congenital myopathy and help advance therapies.
Featured Products: Cas9 mRNA
Have a question? Visit Ask An Expert.

