Manipulating CD38 for a Novel Immuno-Oncology Therapy
Acute myeloid leukemia (AML) is the most common adult acute leukemia, affecting 80% of this patient population. However, the overall 5-year survival rate remains fairly low, at around 29%. Additional therapies are needed to improve the outcome of this disease.
Immunotherapies are based on manipulating the cells of a patient’s immune system. A variety of cancer-targeting immunotherapies have already reached the clinic, and one of the oldest is the bone marrow transplant, which has been used for decades. Combined with chemotherapy, bone marrow transplants represent the current standard of care for most AML patients. More recently, chimeric antigen receptor (CAR)-T cell and immune checkpoint inhibitor therapies have caused an explosion of research in this area, leading to the development of Immuno-Oncology as an independent field.
In CAR-T therapy, a patient’s T cells are genetically altered in a laboratory before being returned to the patient, where they kill cancer cells. A newer CAR immunotherapy is CAR-natural killer (NK) cell therapy, which uses genetically altered natural killer cells rather than T cells to target a patient’s cancer cells. However, NK cells may attack neighboring NK cells (NK cell fratricide), which represents an obstacle to CAR-NK therapy success. Due to lack of an ideal target, development of an immunotherapy for AML has also been challenging. While immunotherapies are in development for AML, there are no currently approved CAR-NK therapies for this disease.
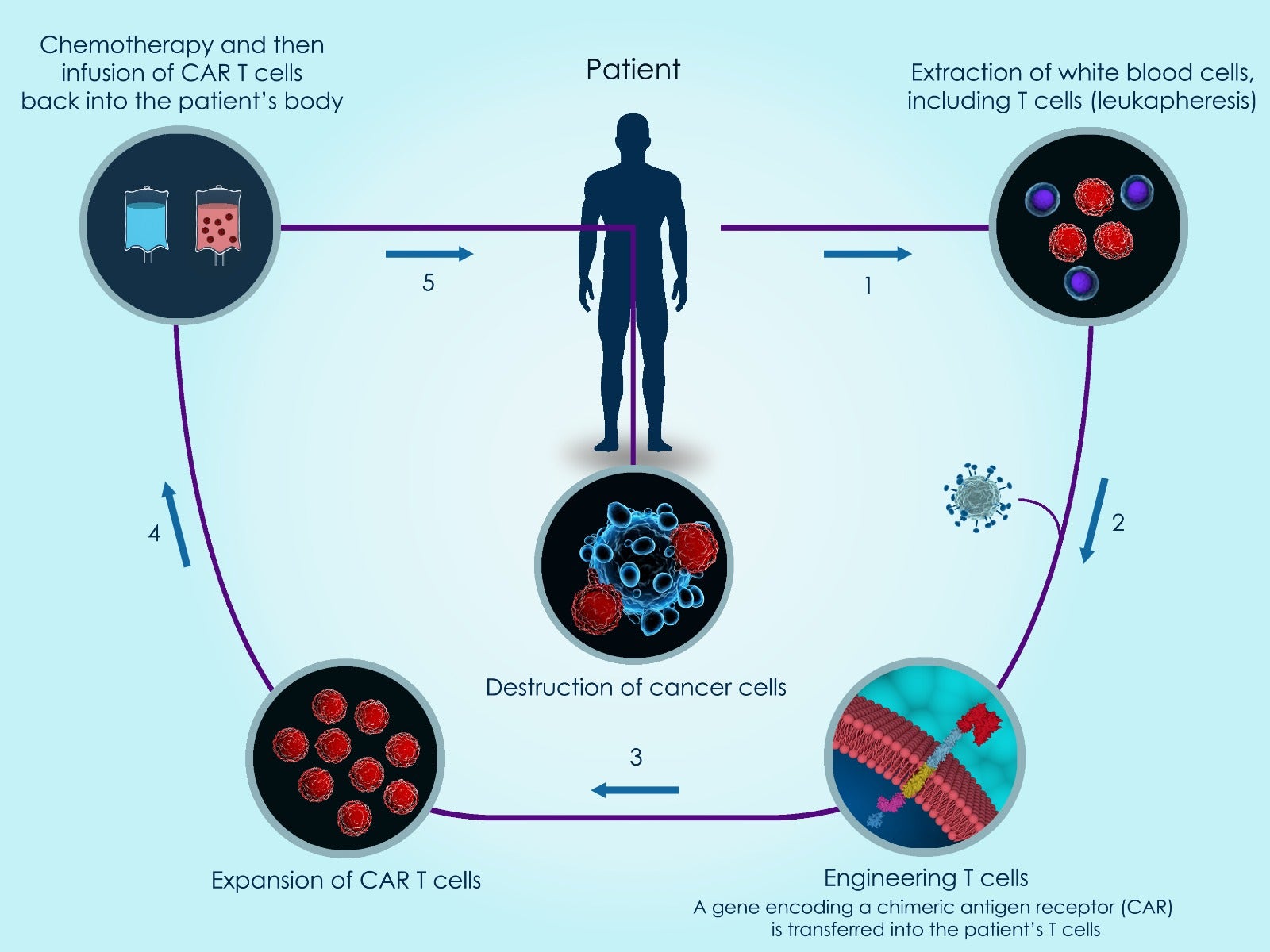
A recent paper published in Haematologica designed and evaluated a novel CAR-NK therapy for AML using CD38 protein. To investigate the feasibility of a CAR-NK approach for AML, the scientists applied a previously designed, affinity-optimized CD38 CAR. First, CRISPR-Cas9 technology was used to reduce endogenous CD38 expression in NK cells. After NK cell expansion in the lab, the affinity-optimized CD38 CAR was expressed to achieve malignant cell targeting, which was accomplished by electroporating a custom-synthesized CD38 CAR mRNA from TriLink BioTechnologies into primary NK cells. These genetically engineered CD38 CAR-NK cells showed reduced propensity for cell fratricide. When co-cultured with bone marrow mononuclear cells from AML patients, the CD38 CAR-NK cells demonstrated enhanced cell killing when compared to mock-electroporated NK cells. These studies employed nine different AML patient samples with varying degrees of CD38 endogenous expression and different AML molecular subtypes.
Overall, this paper showed that replacement of endogenous CD38 and expression of an affinity-optimized CD38 CAR on NK cells could reduce NK cell fratricide and successfully target AML cells. These data support the further clinical development of an affinity-optimized CD38 CAR-NK cell therapy in acute myeloid leukemia.
Although somewhat intricate, this work demonstrates that CD38 could serve as a target for CAR-NK immunotherapy optimization against AML. A remaining question is whether CAR-NK cell therapies have the ability to target the leukemic stem cells believed to cause disease relapse. Most likely, a combination of personalized immunotherapy with chemotherapy will best improve disease prognosis in AML. Timely investigation of affinity-optimized CD38 CAR-NKs will hopefully address the unmet need for AML therapeutics.
Featured product: Custom mRNA synthesis
Article Reference: Haematologica. 2020 Dec 30;Online ahead of print. doi: 10.3324/haematol.2020.271908


