
Restoring Mitochondrial Health after Cellular Stress
Published last month in Cell, Harvard researchers reported that mitochondrial deacetylase, Sirtuin 3 (SIRT3), acts quickly and dynamically to reset acetylation through dissociation with ATP synthase. SIRT3 is known to target molecules that participate in oxidative metabolism, generating the electrons necessary for restoration of membrane potential. The authors hypothesize that this mechanism may act as a means to recover membrane potential after transient cellular stress, before relying upon mitophagy. This finding was identified through a comprehensive analysis of the mitochondrial sirtuin network, which is composed of SIRT3–5.
Sirtuins are a family of proteins that are perhaps most famous for their metabolic role promoting longevity. However, it is becoming more apparent that this family of enzymesexerts a wide range of biological functions, many of which are influenced by their cellular localization ( Figure 1).
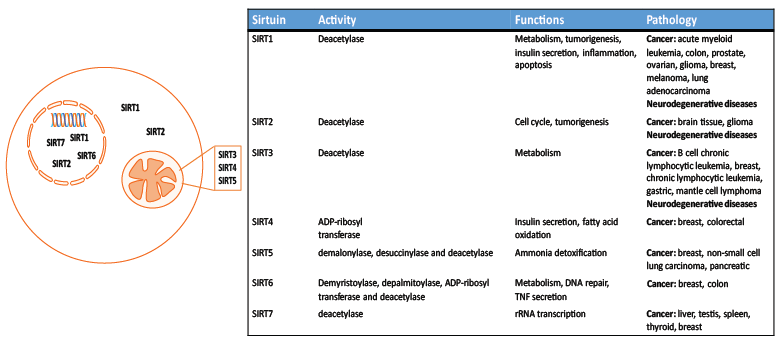
While SIRT3 is the most widely studied mitochondrial sirtuin, the authors noted, "Surveys of the mitochondrial proteome revealed that a surprisingly large number of mitochondrial proteins are acetylated or succinylated. However, our global understanding of the sirtuin-substrate relationships is limited, and only a fraction of mitochondrial are thought to be mediated by SIRT3."
Indeed, they found that SIRT3–5 associate with a number of functional modules important for cellular processes such as mitochondrial homeostasis, transcription, and protein synthesis.
However, it was the novelty of the discovery that SIRT3 acts as a molecular switch in response to cellular stress that prompted additional experiments. The research team was able to demonstrate that SIRT3 is sequestered by ATP synthase under normal physiological conditions, but is released upon membrane depolarization during cellular stress. SIRT3 is then available to quickly reset protein acetylation, helping to restore membrane potential.
The authors then used mutant cell lines generated using the TriLink Cas9 Nickase mRNA to identify specific subunits involved in the interaction. This set of experiments revealed that pH-sensitive SIRT3 release requires H135 in the ATP5O subunit of ATP synthase. This identifies a mechanism to quickly recover membrane potential that had not previously been described.
"In sum, the study reveals a comprehensive mitochondrial sirtuin network, thereby uncovering the diversity and complexity of the sirtuin functions within the mitochondria. This knowledge provides a roadmap for future studies defining substrates for sirtuins and new areas of mitochondrial biology connected to sirtuin function," the authors concluded.
Featured Product: Cas9 Nickase. TriLink also offers high quality Cas9 mRNA as well as reporter genes, such as EGFP and FLuc mRNA. Custom mRNA synthesis is also available from µg to gm scales.

