
Targeted mRNA Delivery through Novel Polymeric Nanoparticles Confirmed using TriLink EGFP
April 2017
Though delivery of nucleic acids has been studied for decades, emerging interest in therapeutic messenger RNAs (mRNAs) has led to renewed interest in biomaterials and nanotechnology platforms designed specifically for mRNA delivery. Despite many unique advantages of mRNA, its delivery has been challenging due to its large size, negative charge, and susceptibility to enzymatic degradation
Of the biomaterials tested, lipid nanoparticles are perhaps the most well-characterized and variations of lipid nanoparticles constitute a large portion of commercially available options. However, although these cationic nanoparticles have been very effective in vitro, in vivo studies have shown that they can be quickly eliminated by the mononuclear phagocyte system.
Alternatively, polymeric nanoparticles contain several qualities that are advantageous for delivery of mRNA, including favorable electrostatic interactions with negatively charged nucleic acids. Though the optimization can be somewhat challenging, the polymeric nanoparticles can form effective delivery vehicles that are easily modified for increased functionality such as ligands for tissue targeting or molecules for pH-responsiveness.
With this in mind, scientists recently published research in Molecular Therapy that sought to optimize polymeric nanoparticles for mRNA delivery. They complexed mRNA with variations of poly(ethylene glycol) (PEG)-polylysine (PLys) (thiol) and poly(N-isopropylacrylamide) (PNIPAM)-PLys(thiol) and later added Arg-Gly-Asp peptide to increase targeting efficiency (Figure 1).
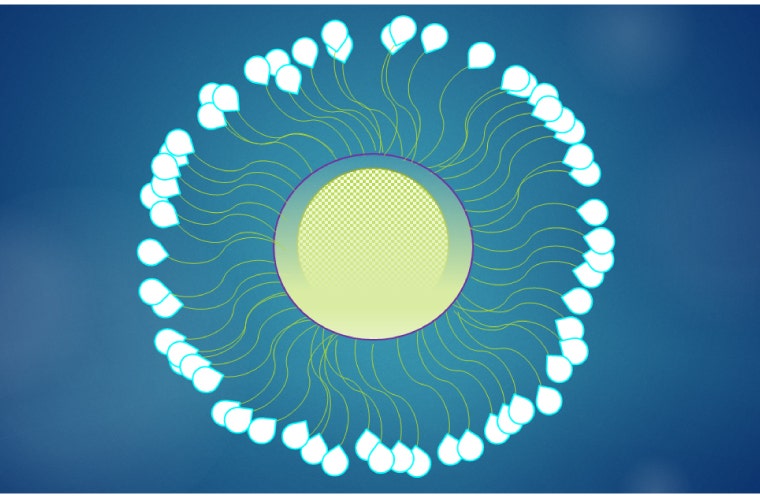
Initial experiments demonstrating structure and function in various environments showed that the PEG-PLys nanoparticles were unable to retain their architecture when challenged with anionic dextran, a molecule used to simulate the negatively charged species or structures within the cellular environment. However, disulfide crosslinking substantially increased the integrity of the nanoparticle under these conditions and the top performing combination was PEG/PNIPAM-PLys(SH). This polymeric nanoparticle also performed well when subjected to fetal bovine serum.
“Our results suggested the proposed formulation of PEG/PNIPAM-PLys(SH) could serve as an appreciable mRNA delivery vehicle to protect mRNA from degradation in the harsh biological milieu,” noted the researchers.
To promote systemic tumor targeting and cellular uptake efficiency, they then conjugated an Arg-Gly-Asp peptide (cRGD) to the nanoparticles, which increases specific interactions with receptors that are overexpressed on the membrane of certain tumor cells,and evaluated cellular uptake and expression by encapsulating the TriLink EGFP mRNA labeled with Cy5 (Figure 2). They found that the cRGD conjugated nanoparticles significantly increased cellular uptake and expression in vitro. Importantly, they were able to confirm that the nanoparticles withstood systemic circulation after tail vein injection and were taken up and mRNA expressed in the targeted tumors in vivo.

“Ultimately, the proposed nanoformulation demonstrated enhanced systemic mRNA expression in tumors. These results validate the physicochemical-based approach to engineering for systemic mRNA delivery systems for tumor-targeted gene therapy. The future for mRNA therapeutics is promising” concluded the researchers.
Though this study doesn’t address toxicity or its efficiency compared to competing delivery strategies such as lipid nanoparticles, they were able to successfully demonstrate the potential utility of an emerging technology for mRNA delivery.
Featured Product: eGFP mRNA. TriLink also offers various versions of modified eGFP, including Cyanine 5–labeled eGFP mRNA (5meC, Y).
Have a question? Visit Ask An Expert.
© 2017 TriLink BioTechnologies. | All Rights Reserved

