In recognition of International Plant Appreciation Day (April 13th) and inspired by plantea.com, which asks us all to imagine a world without coffee, pants or toilet paper (or any one of the thousands of plant-derived products used daily), I decided to explore the world of biomass in further detail. In this post, we’ll discuss:
- The Controversial World of Ethanol Mandates
- Algae: Amazing Single-cell Plants May be the Key to Renewable Energy
- Loving Lignin: High-margin Chemicals from Biomass Waste
Over the past year or so I’ve collected quite a stack of journal publications and news articles—yes, all printed on paper—covering various aspects of using bioengineering—loosely speaking—to produce much needed commodities or important but scarce compounds. Some are needed on humongous scales, thus leading to other engineering challenges, but all at costs which are either competitive or, ideally, much lower than that from current sources. This is the so-called “economic calculus” that virtually every manufactured product must confront.
Frankly, I did not have an easy time trying to decide what to focus on for this post, as all of the items in the stack had intriguing titles, such as those listed below. I’ve linked each one to the source for you to peruse later, should you wish:
- Ethanol push taking a great toll on the environment (San Francisco Chronicle)
- Florida plant is first to produce advanced ethanol (The Wall Street Journal)
- Biofuels beyond ethanol from nonedible plants (The Wall Street Journal)
- Can’t wait 300 million years? Solazyme can produce oil in a few days (Fueling Growth)
- A side trip on the road to clean fuel: algae to energy (The New York Times)
- Microbial production of short-chain alkanes (Nature)
- What happened to biofuels? (The Economist)
- Biomass bonanza: renewable chemical production (Chemistry World)
My choices reflect the increasing shift from the perhaps ill-conceived corn-to-ethanol mandates advocated by Presidents Bush and Obama, to hopefully more realistic goals utilizing non-edible plants or algae to produce “gasoline-like” molecules. I also decided to explore some of the more interesting pursuits of higher margin (i.e. more profitable) chemicals that are useful for diverse purposes, such as anticancer drugs and “environment friendly” bioerodible replacement for conventional plastics derived from oil.
By the way, there are plenty of nucleic acid-related technologies underlying all of these topics, but those are not the focus of my comments in this post, which instead deals with broader stories of general interest.
The Controversial World of Ethanol Mandates
Ethanol (ethyl alcohol, CH3CH2OH) is classified as being either “synthetic ethanol” that is produced chemically from something called syngas, with which you are likely unfamiliar—as I was—or “bioethanol” that has been made for millennia through biological fermentation of sugars from grains and fruits by yeasts, with which you are likely familiar—as I am, and enjoy in adult beverages.
When you have time later, and if you are so inclined, click here to read about the history and details of US energy policies (and politics) prompted by the “1973 oil crisis”—which I’m old enough to have experienced—waiting in long lines at gas stations, hoping there would be some for my car when I got to the pump. One statement therein that caught my attention—and with which I agree—is that there is “criticism that federal energy policies since the 1973 oil crisis have been dominated by crisis-mentality thinking, promoting expensive quick fixes and single-shot solutions that ignore market and technology realities. Instead of providing stable rules that support basic research while leaving plenty of scope for American entrepreneurship and innovation, congresses and presidential administrations have repeatedly backed policies which promise solutions that are politically expedient, but whose prospects are doubtful.”
Here’s where corn-to-ethanol comes in. With the Iowa political caucuses on the horizon in 2007, presidential candidate Barack Obama made homegrown corn a centerpiece of his plan to slow global warming. And when President George W. Bush signed a law that year requiring oil companies to add billions of gallons of ethanol to their gasoline each year, Bush predicted it would make the country "stronger, cleaner and more secure." That quote in the San Francisco Chronicle is followed by these harsh but apparently true criticisms:
But the ethanol era has proven far more damaging to the environment than politicians promised and much worse than the government admits today.
As farmers rushed to find new places to plant corn, they wiped out millions of acres of conservation land, destroyed habitat and polluted water.
 Environmentalist Craig Cox, above, says the push to plant corn for ethanol is damaging land and water. Photo: Charlie Riedel, Associated Press (taken from sfgate.com).
Environmentalist Craig Cox, above, says the push to plant corn for ethanol is damaging land and water. Photo: Charlie Riedel, Associated Press (taken from sfgate.com).
The consequences are so severe that environmentalists and many scientists have now rejected corn-based ethanol as bad environmental policy. But the Obama administration stands by it, highlighting its benefits to the farming industry rather than any negative impact.
The government's predictions of the benefits have proven so inaccurate that independent scientists question whether it will ever achieve its central environmental goal: reducing greenhouse gases. "This is an ecological disaster," said Craig Cox with the Environmental Working Group, a natural ally of the president that, like others, now finds itself at odds with the White House.
Are Bioethanol and Other Biofuels Really Delivering on their Promise?
An in-depth analysis in The Economist points out that bioethanol made from edible plants, such as corn, and biodiesel made from vegetable fat are first-generation biofuels that have drawbacks that extend beyond the negative environmental impact mentioned above.
They are made from plants rich in sugar, starch or oil that might otherwise be eaten by people or livestock. Ethanol production already consumes 40% of America’s corn harvest and a single new ethanol plant in Hull, England is about to become Britain’s largest buyer of wheat. Ethanol and biodiesel also have limitations as vehicle fuels, performing poorly in cold weather and capable of damaging unmodified engines.
According to the The Economist, overcoming these limitations is being addressed by dozens of start-up companies aimed at developing second-generation biofuels. They hoped to avoid the “food versus fuel” debate by making fuel from non-edible (e.g. cellulosic) biomass feedstocks with no nutritional value, such as agricultural waste or fast-growing trees and grasses raised on otherwise unproductive land. Other firms planned to make “drop in” biofuels that could replace conventional fossil fuels directly, rather than having to be blended in.
Unfortunately, the biofuels utopia didn’t pan out—start-ups went bust, surviving companies scaled back their plans and, as the price of first-generation biofuels rose, consumer interest waned. Furthermore, the spread of “fracking” unlocked new oil and gas reserves that provided an alternative path to planned USA energy independence. By 2012 America’s Environmental Protection Agency (EPA) had slashed the 2013 target for cellulosic biofuels to just 53 million (m) liters vs. the planned 3,800m liters—a whopping 98.6% decrease!
The article continues by asking and answering the question: what went wrong?
Basically, three challenges need to be overcome to provide viable second-generation biofuel:
- break down woody cellulose and lignin polymers into simple plant sugars
- convert those sugars into drop-in fuels to suit existing vehicles chemically or biochemically
- do all this on a humongous scale—and, ah, it has to be cheap
And thus comes the real challenge—producing biofuels at a reasonable cost. Oil and gas giant Shell reportedly had ten advanced biofuels projects that are now defunct because they worked in the lab but couldn’t be cost effectively scaled up.
The optimism of five years ago may have waned, but efforts to develop second-generation biofuels continue. Half a dozen companies are now putting the final touches on industrial-scale plants and several are already producing small quantities of second-generation biofuels. Some even claim to be making money doing so.
On the bright side, three plants in America were reported to start producing cellulosic ethanol from waste corn cobs, leaves and husks in 2014: POET-DSM Advanced Biofuels (75m liters) and DuPont (110m liters), both in Iowa, and Abengoa (95m liters) in Kansas. The first company to produce ethanol using enzymes on an industrial scale is Beta Renewables, a spin-off from Chemtex, an Italian chemical giant. An 80m-liter cellulosic ethanol plant near Turin has been running at half capacity since the summer of 2013, using straw from nearby farms. It will run on corn waste in the autumn, rice straw in the winter and then perennial eucalyptus in the spring. The plant claims to be running at a profit but only with local, cheap feedstocks.
Other biofuels companies are continuing to pursue drop-in fuels. You might be wondering why given the challenges previously discussed. One attraction is that drop-in fuels are less susceptible to changing political whims compared with ethanol, the demand for which depends to a large extent on government mandates that it be blended into conventional fuels. Another is that drop-in fuels are commonly made with sugar as a feedstock, either conventionally sourced or cellulosic, and sugar is widely available and easily transported.
The next few years will be an exciting time in the pursuit of second-generation biofuels. With several plants expected to come on line during 2015 and 2016, I’ll be paying close attention to this area to see if the food versus fuel debate heats up or fizzles out.
Algae: Amazing Single-cell Plants May be the Key to Renewable Energy
Can’t wait 300 million years? Solazyme can produce oil in a few days. This is the catchy title of an article by Jeff Benzak at Fueling Growth.org, featuring Solazyme’s use of algae to produce oil. Details of how this came about and how it’s done are better told in a lengthy story reported by Diane Cardwell in The New York Times that I have drawn upon here. Fittingly for International Plant Appreciation Day, you’ll learn that the trick is to use single-celled plants!
Friends since college, Jonathan S. Wolfson (law and business degrees) and Harrison F. Dillon (genetics PhD and law degrees) often mused about using biotechnology to create renewable energy—“delusional rantings” according to Mr. Wolfson. Then Mr. Dillon found algae, and delusional became real. More specifically microalgae, which are a large and diverse group of single-celled plants, produce a variety of substances, including oils, and are thought to be responsible for most of the fossilized oil deposits in the earth. These, it seemed, were micro-organisms with potential. With prodding, they could be re-engineered to make fuel.
 Jonathan Wolfson (left) and Harrison Dillon gaze into the future of algae. Credit : Chronicle/Michael Macor. Taken from cleantechrepublic.com via Bing Images.
Jonathan Wolfson (left) and Harrison Dillon gaze into the future of algae. Credit : Chronicle/Michael Macor. Taken from cleantechrepublic.com via Bing Images.
So, according to the article, in 2003 Mr. Wolfson packed up and moved from New York to Palo Alto, California, where Mr. Dillon lived. They started a company called Solazyme. In mythical Silicon Valley tradition—à la Steve Jobs and Steve Wozniak starting Apple in Job’s Palo Alto garage—they worked in Mr. Dillon’s garage, growing algae in test tubes. And they found a small knot of investors attracted by the prospect of compressing a multimillion-year process into a matter of days.
Now, a decade later—and located in South San Francisco only a short walk from genetic engineering pioneer Genentech—they have released into the marketplace their very first algae-derived oil produced at a commercial scale. However, the destination for this oil—pale, odorless and dispensed from a small matte-gold bottle with an eyedropper—is not gas tanks, but the faces of women and men worried about their aging skin! Sold under the brand name Algenist, the product, sold for $79 for a one-ounce bottle, would seem to have nothing in common with oil refineries and transportation fuel.
It turns out that Algenist and other niche products, such as healthier low-fat food ingredients, are sold at premium prices and may be helping to finance Solazyme’s scale-up of drop-in biofuels. This additional revenue source may be enabling the company to get past the point where so many other clean-tech companies have run out of gas: the so-called “Valley of Death” where new businesses stall trying to shift to commercial-scale production.
Wolfson and Dillon had sold investors on an energy business, not one that made cosmetics, nutritional supplements and soap. They had also told their board that they would be able to make fuel through photosynthesis, a process then considered “sexy,” Mr. Wolfson is quoted as saying in the Times. That’s because the sunlight that would fuel the algae’s growth was free; other methods of goosing the algae included adding food sources like sugar. But growing algae where they could get enough sunlight required huge ponds of water. After “genetic tinkering” with the algae, other processes have been developed, and continuing cost-effective scale-up is being demonstrated.

In June 2012, Solazyme announced the successful commissioning of its first fully integrated biorefinery (IBR) in Peoria, Illinois, to produce algal oil. The IBR was partially funded with a federal grant that Solazyme received from the U.S. Department of Energy (DOE) in December 2009 to demonstrate integrated commercial-scale production of renewable algal-based fuels. The demonstration / commercial-scale plant will have a capacity of two million liters of oil annually, and was said to provide an important platform for continued work on feedstock flexibility and scaling of new tailored oils into the marketplace.

Solazyme currently works with Chevron, UOP Honeywell, and additional industry leading refining partners, to produce SoladieselRD® renewable diesel, SoladieselHRF-76® renewable diesel for ships, and Solajet® renewable jet fuel for both military and commercial application testing.
In 2010, Solazyme delivered over 80,000 liters of algal-derived marine diesel and jet fuel to the U.S. Navy, constituting the world's largest delivery of 100% microbial-derived, non-ethanol biofuel. The company was subsequently awarded another contract with the U.S. Department of Defense for production of up to 550,000 additional liters of SoladieselHRF-76®.
While this fuel-producing capacity is only “a tiny drop in the barrel” relative to billions of gallons of fuels used globally, even a doubtful critic would have to admit that the Peoria plant represents a giant step forward relative to Dillon’s Palo Alto garage!
Biomass Bonanza
“Companies have put biofuels on the back burner to aim for higher margin chemicals,” according to Emma Davies’ presumably pun-intended, lead-in sentence of her excellent article in Chemistry World that I draw from here.
Tom Welton, who is head of chemistry at Imperial College London, UK, views lignin—the ‘really hard stuff’ that protects plants from biological attack—as a valuable source of renewable speciality chemicals, notes Davies. She adds that his group has found a way to extract it from lignocellulosic biomass using ionic liquids—novel ‘liquid salts’—and has plans to make polymers using the technology.
In industrial biochemical and biofuel production, lignin is commonly viewed as little more than waste, sometimes burned to generate energy. Instead, the focus is on biomass cellulose, a polymer composed of repeated units of glucose, locked deep within the complex matrix of lignin and polysaccharides that make up lignocellulose.
Davies adds that “the biofuel bubble has not burst, but it was deformed by the fact that oil prices have not risen as quickly as predicted several years ago.” Consequently, efforts have been redirected to find valuable markets outside of fuels wherein lower scales are applicable and don’t need as much capital investment.
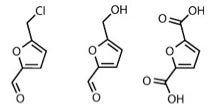
One success story involves a process that uses hydrochloric acid and gentle heat to digest raw biomass to produce high yields of 5-(chloromethyl) furfural (CMF), a starting point for two compounds with a growing market: 5-(hydroxymethyl)furfural and furan-2,5-dicarboxylic acid. The latter is used to make poly(furan-2,5-dicarboxylic acid) known as PEF, a furan version of PET (polyethylene terephthalate), which can be used to make plastic bottles.
Thus soft drinks are partly responsible for PEF’s expanding market. In 2011, Coca-Cola announced multi-million dollar partnership agreements with three biotechnology companies, Virent, Gevo and Avantium, to ‘accelerate development’ of the commercial solutions for bottles made solely from plant-based materials. While Gevo and Virent focus on PET made from bio-based paraxylene, Dutch company Avantium uses plant-based materials as feedstocks to produce PEF; it also has a bottle deal with French food company Danone.
Such chemical-based processes have clear advantages over the alternative of using selected microorganisms to digest biomass to produce chemicals. The clear advantage is time, because no matter how clever your bugs get they still need time to process whatever it is they are working on—usually days. Also, microorganisms don’t work for free. If you have a glucose molecule, two of those six carbons end up as carbon dioxide. A chemical process has a lot of potential advantages, including the possibility to keep all of the carbon.
Lovin’ lignin
The process to convert raw biomass to CMF leaves behind lignin. Davies article quotes a researcher as saying that ‘if anybody were ever to come up with a really simple economic way of getting some very useful molecules—simple aromatic hydrocarbons—in a really good yield from lignin, that would be worth a Nobel prize’. The researcher adds, however, that ‘it’s an enormous challenge because it’s such complicated stuff and so highly oxidized. Oxygens are attached directly to the benzene ring. That’s a very powerful bond which is difficult to break.’
Davies goes on to quote Art Ragauskas from the Georgia Institute of Technology, in Atlanta. ‘There is a lot of interest in what can be done with lignin because the cellulosic ethanol plants generate a lot of it. We have always argued that, since lignin is 20–30% of biomass, then you cannot afford just to say we’ll leave it there and burn it for energy.’
Ragauskas is working on ways to overcome the significant problem of biomass ‘recalcitrance’, the way that lignocellulose’s structural complexity makes it quite resistant to enzymatic hydrolysis used to unlock the cellulose. ‘Recalcitrance is probably the largest sole contributor to the capital and operating costs of converting lignocellulosics to biofuels,’ he says.
‘Five to six years ago, when people talked about recalcitrance, some were skeptical that it would be addressable,’ he says. Now the situation is improving. Ragauskas works with biologists from the BioEnergy Science Center looking at transgenic plants that can reduce recalcitrance, while also carrying out in-depth structural studies to gain an insight into the effects of different types of biomass pre-treatment.
It’s Ionic
According to Davies, Imperial’s Welton is excited about his group’s work to extract lignin from biomass using ionic liquids. The discovery was made by chance by PhD student Agniezka Brandt, who was working on dissolving biomass using ionic liquids. When some of the reactions did not work as well as others, Welton asked Brandt to find out why. ‘I’ve selectively taken the lignin out,’ revealed Brandt after further investigation. ‘We were lucky but we had prepared minds. This really does look promising as an approach,’ says Walton.
Post-petroleum Polymers
Meanwhile, Welton has a new project to investigate ionic liquid biorefining of lignocellulose to sustainable polymers, sponsored by the UK’s Engineering and Physical Sciences Research Council. He outlines three ‘synthetic challenges’ for renewables. The first, he says, is to get a new source of a compound in use today. Second is to make materials with properties that match those of chemicals currently in use, and third is to create large volumes of chemicals that have new properties and then discover what the chemicals could be used for.
Welton is particularly taken with the second challenge, which his polymer project targets. ‘It’s not the sophisticated chemicals that will be a problem [in a post-petroleum era>
. We will find a way of making all the sophisticated chemicals that we want, as we do now,’ he says. ‘We really need to think about the low-cost, high-volume, high-mass materials.’ He looks at all the plastic items on his desk, from the folder that holds his lecture notes to his pen holder. ‘What are we going to make those kind of things from, the really mundane stuff that we take for granted that is all around us?’ he asks.
‘It has to be very simple, based on what’s available and the sophistication of the synthesis has to be really, really good because the margins will be so tiny. So that puts an amazing discipline on the chemistry that you’re going to have to invent,’ says Welton. ‘For me, as a chemist, I find that interesting.’
Big Biomass Breakthrough
In closing, I should add that a research group at The Department of Chemical and Biological Engineering at the University of Wisconsin-Madison, Wisconsin, reported in Science in January 2014 the first nonenzymatic sugar production from biomass using biomass-derived γ-valerolactone. They achieved laboratory-scale production of soluble carbohydrates from hardwood and softwood at high yields (70 to 90%) in a solvent mixture of biomass-derived γ-valerolactone (GVL), water, and dilute sulfuric acid. GVL completely solubilizes to promote the biomass, including the lignin fraction. The carbohydrates can be recovered and concentrated by extraction from GVL into an aqueous phase by addition of NaCl or liquid CO2. The researchers claim that “this strategy is well suited for catalytic upgrading to furans or fermentative upgrading to ethanol at high titers and near theoretical yield. We estimate through preliminary techno-economic modeling that the overall process could be cost-competitive for ethanol production, with biomass pretreatment followed by enzymatic hydrolysis.”
Hopefully the cutting edge research touched upon in this posting will accelerate the world’s ability to transition from dwindling petroleum-based resources to environmentally safe renewable resources.
Discussions around biofuels always seem to evoke a number of vast and varied opinions. I hope you’ll share yours in the comments section below.







