- Current “CRISPR Craze” for DNA Editing is Catalyzing Creativity
- Early CRISPR Innovator Feng Zhang Now Reports Targeting RNA
- This New “C2c2” System has Specificity Issues But is Nevertheless Promising
Just when you thought that the “CRISPR craze” would soon transition from the fundamental discovery phase to the improvements phase, something entirely new for CRISPR has come along. That something, recently published by Feng Zhang and others in venerable Science magazine, targets RNA instead of DNA. Consequently, this may lead to transient vs. permanent editing, as well as other RNA- vs. DNA-based applications.
Before further commenting on this exciting new RNA-targeting approach using CRISPR, here are a few snippets about the original DNA version of CRISPR to set the stage, and substantiate my tongue-in-cheek referral to the craze about it.
Backstory
 Taken from igtrcn.org
Taken from igtrcn.org
Editing with CRISPR, which is short-form for CRISPR-Cas9, uses sequence-specific guide RNA (gRNA) to target DNA for cutting by Cas9 nuclease, as depicted below. Guide RNA and Cas9 can be introduced into cells either encoded in a vector or as synthetic gRNA and synthetic Cas9 mRNA, which TriLink offers in either wild-type or base-modified forms of Cas9 mRNA. CRISPR for genome editing was publicly described in Science in 2012 by co-corresponding authors Jennifer A. Doudna, a biologist at the University of California, Berkeley, and the French microbiologist Emmanuelle Charpentier. But Feng Zhang, at the Broad Institute, was first to obtain a patent on the technique.
Not surprisingly, given the financial potential for DNA editing by CRISPR, which has been called the ‘biotech discovery of the century,’ there is ownership litigation. This dispute is getting rather ugly, if you will, according to an article in Science titled Accusations of errors and deception fly in CRISPR patent fight.
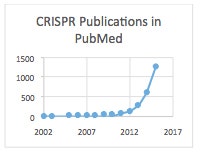 Potential financial gain aside, PubMed stats I found clearly substantiate the craze factor in numeric terms: >4,000 publications to date with a rapidly increasing trajectory, i.e. ~600 in 2014 and ~1,200 in 2015, which is an average of roughly 4 publications every day in that year!
Potential financial gain aside, PubMed stats I found clearly substantiate the craze factor in numeric terms: >4,000 publications to date with a rapidly increasing trajectory, i.e. ~600 in 2014 and ~1,200 in 2015, which is an average of roughly 4 publications every day in that year!
By the way, in the chart above that I made for CRISPR publications in PubMed, there was only one report in 2002, which was the first publication to identify CRISPR. These Dutch investigators used computer analysis to find a novel family of repetitive DNA sequences that is present among both domains of the prokaryotes (Archaea and Bacteria), but absent from eukaryotes or viruses. They noted that “[t>
his family is characterized by direct repeats, varying in size from 21 to 37 bp, interspaced by similarly sized non-repetitive sequences. To appreciate their characteristic structure, we will refer to this family as the clustered regularly interspaced short palindromic repeats (CRISPR).”
 Taken from youtube.com
Taken from youtube.com
CRISPR was also selected as 2015 Science Breakthrough of the Year, and is featured in an interesting YouTube video that is definitely worth watching, in my opinion.
Enough said for CRISPR editing of DNA, let’s move on to RNA editing with CRISPR that offers a fundamentally different editing approach: whereas DNA editing makes permanent changes to the genome of a cell, CRISPR-based RNA-targeting approach may allow researchers to make temporary changes. Moreover, this can be adjusted up or down, and may one day provide greater specificity and functionality than existing methods for RNA interference (RNAi) using either siRNA or antisense oligos.
CRISPR Targeting RNA

At the risk of seeming to be too trendy, this section heading could have read “Feng Zhang 2.0” in that Zhang at the Broad Institute, along with co-corresponding author Eugene V. Koonin at NIH and uber-famous “Broadster” Eric Langer plus others on a large team, have characterized a new CRISPR system that targets RNA—but not DNA. In their recent Science publication they demonstrated that this new system involves a Class 2 type VI-A CRISPR-Cas effector—aptly abbreviated C2c2 (pronounced “see too, see too”)—that has RNA-guided RNase function.
The researchers originally identified C2c2 in the bacterium Leptotrichia shahii (L. shahii) in a systematic search for previously unidentified CRISPR systems within diverse bacterial genomes. They focused on C2c2 because its sequence contained two copies of a domain called higher eukaryotes and prokaryotes nucleotide-binding (HEPN) that has only been found in RNases. Mutating the putative catalytic site within either of C2c2’s HEPN domains demonstrated that none of the mutated enzyme versions could cut RNA in vitro, suggesting that both HEPN domains are necessary for C2c2 to work.

But C2c2 Cleaves Collateral RNA—a Case for “Lemons into Lemonade”?
Unlike Cas9, which cuts DNA only within the sequence dictated by the CRISPR gRNA, C2c2 was found to make cuts within the target sequence and adjacent, nonspecific sequences. While this collateral cleavage obviously presents a specificity problem, Zhang and his colleagues were able to create a deactivated C2c2 (dC2c2) variant by alanine substitution of any of the four predicted HEPN domain catalytic residues. To me this clever trick is like converting “lemons into lemonade” in that undesired non-specific cleavage is transformed into a programmable RNA-binding protein having potential utility.
For example, the investigators speculate that the ability of dC2c2 to bind to specified sequences could be used in the following ways:
- Bring effector modules to specific transcripts in order to modulate their function or translation, which could be used for large-scale screening, construction of synthetic regulatory circuits, and other purposes.
- Fluorescently tag specific RNAs in order to visualize their trafficking and/or localization.
- Alter RNA localization through domains with affinity for specific subcellular compartments.
- Capture specific transcripts through direct pull-down of dC2c2 in order to enrich for proximal molecular partners including RNAs and proteins.
Listen to Zhang’s Grad Students
While the details of this seminal work published in Science is not easily summarized, its practical implications have been concisely translated, if you will, by first coauthor Omar O. Abudayyeh, and second coauthor Jonathan S. Gootenberg—both graduate student members of the Zhang lab—in three short videos that I encourage you to watch at this link.

Publication protocol generally lists coauthors in order of contribution, so in this C2c2 publication that has many coauthors, these fellows know what they’re talking about because they did lots of the lab work. Congrats to them, Feng Zhang (again), and all of the other contributors.
As always, your comments are welcomed and encouraged.






