- Strong Interest in mRNA Therapeutics Drives Increased Numbers of Delivery Publications
- Novel Charge-Altering Releasable Transporters (CARTs) Undergo “Self-Immolation”
- CARTs Outperform Widely Used Lipofectamine In Vitro and Enable In Vivo Delivery
Devotees of this blog may recall my past post in 2013 titled Modified mRNA Mania, which intentionally used the word “mania” to provoke reading about the trending topic on base-modified mRNA as therapeutic agents. My metrics for this mania were a flurry of scientific publications, patent applications staking out intellectual property, and massive investments by venture capitalists and established pharma companies in mRNA therapeutics startups.
As with antisense, siRNA, and antagomir RNA drugs, efficient delivery is widely recognized as a critical technical challenge to overcome. And, not surprisingly, past lipid-based approaches of various sorts are being reinvestigated for repurposing for mRNA delivery.
The focus of the present blog is a new strategy for mRNA delivery developed by a team of collaborators at Stanford University. Although I’ve chosen to highlight this report by McKinlay et al. in prestigious Proc. Natl. Acad. Sci., a search of PubMed for publications indexed to “mRNA delivery” in the title and/or abstract for the period 2005 to 2017 gave articles that can be perused at this link. The graph shown below supports my characterization of this level of activity as “deluge”-like in that there are more than 100 publications, mostly in the last few years, with 40 to 50 more during 2018, by my estimate.

Challenges for mRNA Delivery
Simply stated, the key challenge associated with the use of therapeutic mRNA is an inability to efficiently deliver functionally intact mRNA into cells. Like all nucleic acid-based drugs, mRNA is a macromolecular polyanion and thus it does not readily cross nonpolar cellular and tissue barriers. Moreover, it is also susceptible to rapid degradation by nucleases and ideally it should be protected during the delivery process, even though some success has been reported using intradermal injection of “naked” unmodified mRNA. Finally, after cell entry, rapid release of mRNA in the cytosol and appropriate association with the protein synthesis apparatus is required for translation.
Each of these is a potential point of failure for functional mRNA delivery. In addition to the challenges associated with complexation, protection, delivery, and release, an ideal delivery system would also need to be synthetically accessible, readily tuned for optimal efficacy, and safe.
Charge-Altering Releasable Transporters (CARTs)
McKinlay et al. have successfully addressed each of the challenges mentioned above by developing a highly effective mRNA delivery system comprising charge-altering releasable transporters (CARTs). Since a picture is worth a thousand words, I’ve reproduced here the diagram used by McKinlay et al. to describe their multistep approach with CARTs, namely complexation (1), intracellular delivery (2), and cytosolic release (3) of mRNA transcripts, resulting in induction of protein expression (4).
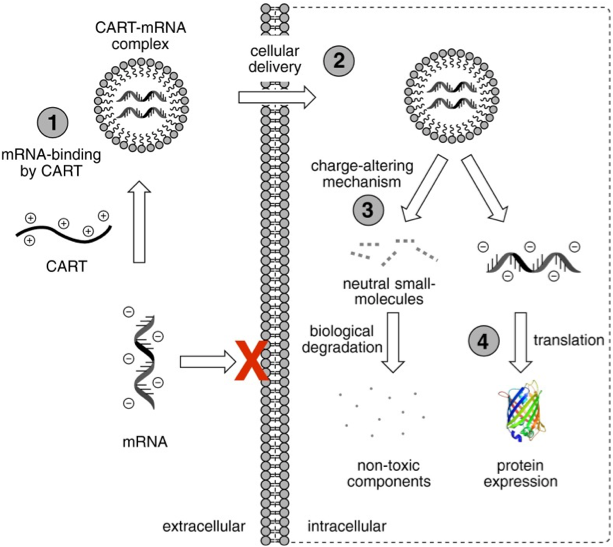 Taken from McKinlay et al. Proc. Natl. Acad. Sci (2017)
Taken from McKinlay et al. Proc. Natl. Acad. Sci (2017)
Readers interested in the clever chemistry that underlies CARTs should consult the publication by McKinlay et al. for details. In brief, these dynamic materials, specifically oligo(carbonate-b-α-amino ester)s (1) shown below function initially as polycations that noncovalently complex, protect, and deliver polyanionic mRNA and then subsequently lose their cationic charge through a controlled degradation to a neutral small molecule (2). The proposed mechanism for this degradation mechanism, which McKinlay et al. refer to as “self-immolative,” is pH-dependent.
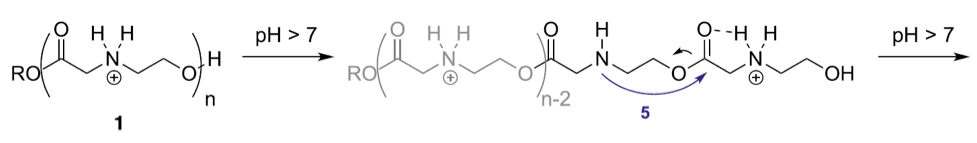

As exemplified below, CARTs for cellular uptake were synthesized with hydrophobic blocks (n = 15) and cationic blocks (n = 12) such that 11b in physiological phosphate buffered saline (PBS) at pH 7.4 undergoes degradation to form 11c and small molecule 2.

These researchers hypothesize that this charge alteration reduces or eliminates the electrostatic anion-binding ability of the originally cationic material, thereby facilitating endosomal escape and enabling free mRNA release into the cytosol for translation. Readers interested in learning more about the complexities of endosomal escape can consult a (free, via Google) book chapter by Uyechi-O'Brien and Szoka titled Mechanisms for Cationic Lipids published in 2003, and a 2012 review by Nguyen and Szoka rhetorically titled Nucleic Acid Delivery: The Missing Pieces of the Puzzle?
Regardless of the actual mechanistic details for CARTs, McKinlay et al. demonstrate the efficacy of these materials to complex, deliver, and release mRNA in various lines of cultured cells including primary mesenchymal stem cells and in animal models, via both intramuscular (i.m.) injection and intravenous (i.v.) administration, resulting in robust gene expression. I’ll briefly outline these findings in what follows; however, the full paper and its supplemental material should be consulted for details.
Incidentally, I’m pleased to add that these CARTs were used to deliver the following base-modified [5-methylcytidine (5meC ) and pseudouridine (Ψ)>
reporter mRNAs and dye-labeled mRNA obtained from TriLink BioTechnologies: Enhanced Green Fluorescent Protein (EGFP) mRNA, Firefly Luciferase (Fluc) mRNA, and Cyanine 5 (Cy5)-labeled EGFP mRNA.
Mechanism of Uptake and Release
Using a Cy5-labeled EGFP mRNA it was determined that the mechanism of cell entry for CART mRNA polyplexes is predominantly endocytic by comparing cellular uptake at 4 °C, a condition known to inhibit endocytotic processes, to normal uptake at 37 °C. Consistent with the expected endocytotic mechanism for ∼250-nm particles, HeLa cells displayed a significant (85%) reduction in Cy5 fluorescence at 4 °C.
Cellular uptake and mRNA translation following treatment with CART/mRNA polyplexes were then directly compared with polyplexes formed with non-immolative oligomers. By delivering a mixture of EGFP mRNA and Cy5-labeled EGFP mRNA, analysis of mRNA internalization and expression can be decoupled and simultaneously quantified: Cy5 fluorescence indicates internalized mRNA, irrespective of localization, and EGFP fluorescence denotes cytosolic release and subsequent expression of mRNA.

This method was used in conjunction with confocal microscopy to compare cellular uptake and mRNA expression of two oligomers, namely, CART D13:A11 (7) and non-immolative, guanidinium-containing D13:G12 (13). Detection included dansylated transporter, Cy5-mRNA, and tetramethylrhodamine (TRITC)-Dextran4400, a stain for endosomal compartments. When cells were imaged 4 h after treatment with CART 7/Cy5-mRNA complexes diffuse fluorescence was observed for both the Cy5 and dansyl fluorophores, indicating that those materials successfully escaped the endosome and dissociated from the polyplexes (i).

The two observed puncta in the dansyl signal (ii) was attributed to some intracellular aggregation of the dansyl-labeled lipidated oligocarbonate blocks, resulting from self-immolative degradation of the cationic segments of CART 7. Diffuse fluorescence from (TRITC)-Dextran4400 was also observed and attributed to endosomal rupture and release of the entrapped dextran.
However, when cells are treated with non-immolative 13/Cy5-mRNA complexes, both the Cy5 and dansyl fluorescence remain punctate and colocalized (iii). These signals also strongly overlap with punctate TRITC-Dextran4400, indicative of endosomal entrapment.
Taken together, according to McKinlay et al., these data strongly suggest that the charge altering behavior of CART 7 enables endosomal rupture and mRNA release, contributing to the high performance of these materials for mRNA delivery.
Applications and Animal Experiments
Oligo(carbonate-b-α-amino ester) D13:A11 7 was evaluated in applications to explore the versatility of CART-mediated mRNA delivery. EGFP mRNA expression following delivery by CART 7 was assayed in a panel of cell lines and compared to widely used Lipofectamine 2000 (Lipo). HeLa cells, murine macrophage (J774), human embryonic kidney (HEK-293), CHO, and human hepatocellular carcinoma (HepG2) cells all showed that the percentage of cells expressing EGFP using the CART 7 was >90%, whereas treatment with Lipo induced expression in only 22–55% of the cells. Importantly, in addition to these various immortalized cell lines, mRNA expression was also observed in primary CD1 mouse-derived mesenchymal stem cells (MSCs) with high transfection efficiency.
In vivo bioluminescence imaging (BLI) enables localization and quantification of expression following mRNA delivery in living animals. To assess the efficacy of CART/mRNA complexes following local (i.m.) or systemic (i.v.) routes of administration, CART 7-complexed Fluc mRNA (7.5 μg ) in PBS (75 μL) was given to anesthetized BALB/c mice in the right thigh muscle. As a direct control, naked mRNA was similarly injected in the opposite flank. D-luciferin was systemically administered i.p. at 15 min before imaging for each time point, and luciferase expression was evaluated over 48 h, starting at 1 h after the administration of mRNA complexes.
As shown here, when Fluc mRNA was delivered with polyplexes derived from 7 into the muscle, high levels of luciferase activity were observed at the site of injection. This expression peaked at 4 h and was still observable after 24 h but barely so after 48 h (see publication for percentages). In contrast, i.m. injection of naked mRNA afforded only low levels of luciferase expression, as measured by photon flux, in all five mice (see publication for percentages).

Following i.v. injections, the localization of mRNA polyplexes in tissues along the reticuloendothelial system pictured here provides many opportunities in inducing immunotherapeutic responses. According to McKinlay et al., spleen localization is “particularly exciting for future studies involving mRNA-based immunotherapy due to large numbers of dendritic and immune cells in that tissue.” Liver localization was also apparent in these animals, and expression in this tissue “may have applicability for treatment of hereditary monogenic hepatic diseases requiring protein augmentation or replacement such as hereditary tyrosinemia type I, Crigler–Najjar syndrome type 1, alpha-1-antityrpsin deficiency, Wilson disease, and hemophilia A and B, or acquired liver diseases such as viral hepatitis A–E and hepatocellular carcinoma.”

Future Perspectives
Rather than paraphrase the future perspectives envisaged by McKinlay et al., here are those views, which to me seem warranted by the promising results summarized above:
“The effectiveness of mRNA delivery using these CARTs represents a strategy for mRNA delivery that results in functional protein expression in both cells and animals. The success of these materials will enable widespread exploration into their utilization for vaccination, protein replacement therapy, and genome editing, while augmenting our mechanistic understanding of the molecular requirements for mRNA delivery.”
As usual, your comments are welcomed.







