- Chinese Team Begins First CRISPR-Based Anticancer Immunotherapy Clinical Study
- US Consortium’s CRISPR Clinical Study OK’d by NIH Panel
- Survey Indicates More Worry than Enthusiasm for Gene-Editing in Babies
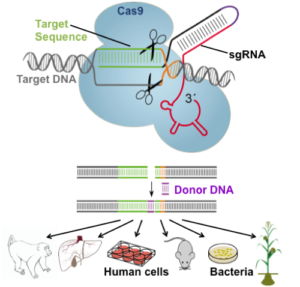 Taken from jeantet.ch
Taken from jeantet.ch
Regular readers of my blog will recall a number of previous postings on gene editing using CRISPR-Cas9 (aka CRISPR), which has rapidly become the hottest trend in nucleic acid-based biotechnology and medical research. That opinion is partly based on the fact that this relatively new technology has already amassed thousands of publications, including 1,250 in 2015 alone! Additional evidence follows from the numerous start-up and established biopharma companies pursuing CRISPR-based therapeutics, which is “the biggest biotech discovery of the century” according to one report.
Now, even more exciting developments for CRISPR are on the horizon. Chinese researchers are poised for the first human clinical trial using CRISPR, and an analogous study in the US awaiting FDA approval. Both of these trials involve forms of cancer immunotherapy, which is a very hot field in itself, and is briefly introduced in the next section.
Cancer Immunotherapy
Cancer immunotherapy is the use of the immune system to treat cancer either actively, passively, or by a combination of these approaches, which exploit the fact that cancer cells often have macromolecules on their surface that can be detected by the immune system (aka tumor-associated antigens, TAAs). Active immunotherapy directs the immune system to attack tumor cells by targeting TAAs, whereas passive immunotherapies enhance existing anti-tumor responses and include the use of monoclonal antibodies, lymphocytes and cytokines.
Numerous antibody therapies have been approved by the FDA and other regulatory authorities worldwide. In contrast, active immunotherapies, which usually involve the removal of immune cells from the blood or from a tumor for reintroduction to the patient, have lagged in such approvals. In fact, the only US-approved cell-based therapy is Dendreon's Provenge®, for the treatment of prostate cancer. A recent series of NY Times articles highlights some powerful examples of cancer immunotherapy, especially from the perspective of interviews with patients and their physicians.
 Taken from howitworksdaily.com
Taken from howitworksdaily.com
For the purpose of this blog, I’ll add that adoptive T-cell therapy is a form of passive immunization by the transfusion of T-cells, which are found in blood and tissue and usually activate when they find “foreign” pathogens. These pathogens can be either infected cells, or antigen presenting cells present in tumor tissue, where they are known as tumor infiltrating lymphocytes. Although these cells can attack the tumor, the environment within the tumor is highly immunosuppressive, preventing immune-mediated tumor death.
As depicted below, T-cells specific to a tumor antigen can be removed from a tumor sample or filtered from blood. Subsequent activation and culturing is performed ex vivo, with the resultant cells being reinfused. Importantly, activation can take place through genetic engineering, such as gene editing by CRISPR.
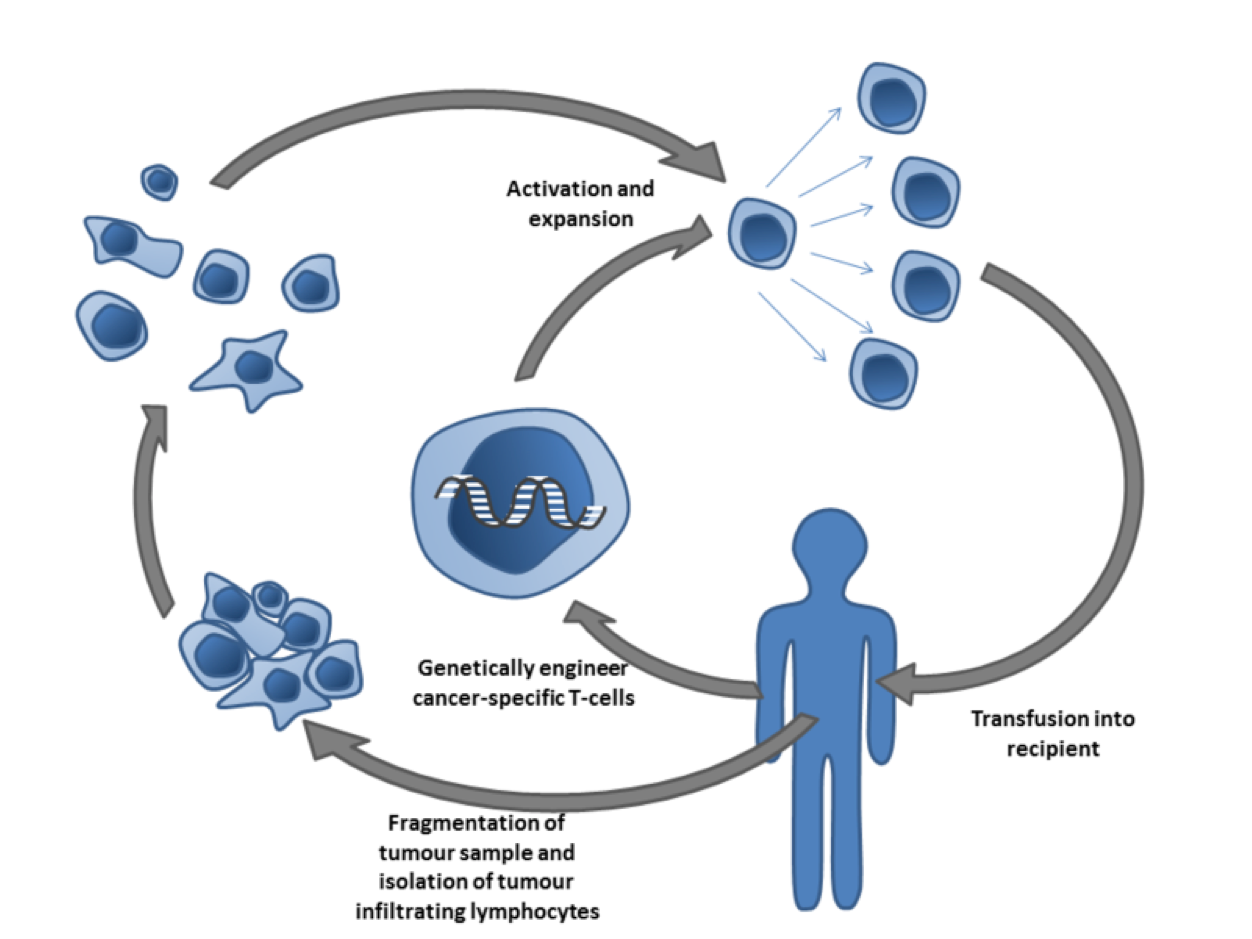 Cancer specific T-cells can be obtained by fragmentation and isolation of tumor infiltrating lymphocytes, or by genetically engineering cells from peripheral blood. The cells are activated and grown prior to transfusion into the recipient (tumor bearer). Taken from Wikipedia.org
Cancer specific T-cells can be obtained by fragmentation and isolation of tumor infiltrating lymphocytes, or by genetically engineering cells from peripheral blood. The cells are activated and grown prior to transfusion into the recipient (tumor bearer). Taken from Wikipedia.org
CRISPR Goes Clinical in China
As I write this post, Chinese scientists at Sichuan University’s West China Hospital in Chengdu will reportedly become the first in the world to inject people with cells modified using the CRISPR gene-editing method. This landmark trial will involve patients with metastatic non-small cell lung cancer who have not responded to chemotherapy, radiation therapy and/or other treatments.
The team will extract T cells from the blood of the trial participants and then use CRISPR to knock out a gene encoding a protein called PD-1 that normally regulates the T cell’s immune response to prevent it from attacking healthy cells. As depicted below, this so-called checkpoint has been targeted by small molecule inhibitors (anti PD-1), but with somewhat limited success so far—notably including failure of a would-be billion-dollar drug (Opdivo®) recently reported by Bristol-Myers Squibb. Nevertheless, the presently envisaged PD-1 gene-edited cells will be amplified in the lab and re-introduced into the patient’s bloodstream to circulate and, hopefully, attack the cancer.

Potential Concerns of CRISPR Clinical Trials
While the Chinese trial may be groundbreaking, it is not without risk. There is concern that the edited T-cells could attack normal tissue as it’s well documented that CRISPR can result in gene edits at the wrong place in the genome. To help mediate this risk, the T-cells will be validated by biotechnology company MedGenCell—a collaborator on the trial—to ensure that the correct gene is knocked out before the cells are re-introduced into patients. Since the primary purpose of this phase one trial is to determine if the approach is safe, participants will be closely monitored for side effects, and researchers will pay close attention to biological markers in the blood.
Some may say that this type of trial in humans is premature, but China has always tried to be a leader in CRISPR research. Carl June, a clinical researcher in immunotherapy at the University of Pennsylvania in Philadelphia explains, ‘China places a high priority on biomedical research.’ And Tetsuya Ishii, a bioethicist in Japan, is quoted in Nature magazine as saying that ‘When it comes to gene editing, China goes first.”
Initial CRISPR Clinical Trial in USA Awaiting Review Board and FDA Approvals
While the Chinese team seems positioned to be the first to conduct a CRISPR clinical trial, the US isn’t far behind. As reported earlier this summer, the Recombinant DNA Research Advisory Committee (RAC) at the NIH has approved an analogous—but genetically more complex—proposal to use CRISPR-edited T cells for cancer immunotherapy. The RAC reviews all proposals for human trials involving modified DNA that are conducted in the United States, and the investigators now have to convince review boards at their own institutions, and the FDA, to allow the trial. This will hopefully be done by the end of 2016.
The proposed trial is a collaborative effort involving the University of Pennsylvania, M.D. Anderson Cancer Center in Texas, and the University of California, San Francisco. This trial will be funded by a $250-million immunotherapy foundation formed in April 2016 by former Facebook president Sean Parker. According to a news item in Nature, The University of Pennsylvania will produce the edited cells, and will recruit and treat patients alongside the collaborating medical centers in Texas and California.
In this study, researchers will perform three CRISPR edits with T cells from patients with several types of cancers: 1. a gene for a protein engineered to detect and target cancer cells will be inserted, 2. a natural T-cell protein that could interfere with this process will be removed and 3. the gene for a protein that identifies the T cells as immune cells and prevents the cancer cells from disabling them will be removed. The researchers will then infuse the edited cells back into the patient.
Both of the studies being performed by the Chinese and US teams are very exciting to me. I will be paying close attention to the progress of these studies over the next few months and hope to report back to you with some very interesting and ground breaking results.
Survey Indicates Public Concern in the US About Gene Editing in Babies
A Pew Research Center pole focusing on concerns about gene editing was recently reported in Nature. The poll surveyed more than 4,700 people in the United States and the results indicate that more people are concerned than enthusiastic about gene editing.
The quadrants below (from 0-100%) show responses to the question, “How worried or enthusiastic do you feel about gene editing to reduce disease risk in babies?”

While these findings speak for themselves, I hasten to add that this same pole also asked “Have you heard or read about this topic?” The following replies (in quadrants from 0-100%) indicate that a substantial percentage of respondents who have not at all read about gene editing nevertheless expressed either worry or enthusiasm for gene editing. Ditto for those who read a little about gene editing.
It would seem, then, that we should take this poll with a grain (or two) of salt as it apparently includes a substantial amount of uninformed opinion. This is not too surprising given the complex nature of gene editing and it’s relatively new position in research. We can see from the results of this poll that a significant amount of education may be needed to garner increased public support for gene editing. Hopefully, the results of the studies highlighted above will begin to pave this road.
Hope for the Best but Expect Complications
This section heading, which summarizes my parting comments about the advent of CRISPR-enabled clinical trials, is based upon my having participated in several decades of research on nucleic acid-based concepts for radically different approaches to traditional small-molecule therapeutics. First was the idea of using chemically modified antisense oligonucleotides to block gene expression, which encountered non-specificity issues, low potency, delivery, and a host of other issues that took two decades to sort through. Then there was the idea of using chemically modified short-interfering RNA (siRNA) for modulating gene expression via RNA interference (RNAi). This, too, encountered similar problems in reaching the clinic. Ditto for anti-microRNAs (antagomirs).
I’m hoping that CRISPR-based therapies meet with success far faster—and prove to be affordable to society. I won’t, however, be surprised if progress is slow—and quite expensive.
Are you excited about potential CRISPR-based therapies? Do you have concerns about their safety and efficacy? Do you believe the general public is ready to accept gene editing therapies? Please share your thoughts as your comments are always welcome.







