- IRESs Are RNA Structures That Allow Translation Initiation to Occur in a 5’ Cap-Independent Manner
- 10–15% of All Human Genes May be Translated via IRESs
- The First Biosensor to Test for the Integrity of Synthetic mRNA Has Been Reported
In human and other types of eukaryotic cells, translation initiation of most mRNAs is mediated by ribosome scanning, in which the translation initiation complex recognizes and binds to the cap structure at the 5′-end of the mRNA. The ribosome then scans the mRNA in a 5′ → 3′ direction, through 5′-untranslated regions (UTRs) that are typically 50–100 nt long, until the first AUG start codon within an optimal context is encountered. Interested readers can refer to additional details elsewhere.
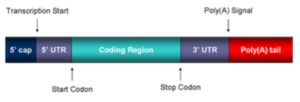 Mature mRNA structure. Taken from commons.wikimedia.org and free to use.
Mature mRNA structure. Taken from commons.wikimedia.org and free to use.
In 1988, Jang et al. discovered that encephalomyocarditis virus (EMCV) RNA is translated by a distinctly different mechanism in which ribosomes initiate translation on highly structured regions of RNA located within the 5′-UTR. These regions were named internal ribosome entry sites (IRESs). The same year, in a publication in Nature by Pelletier and Sonenberg, it was suggested that that this novel, IRES-mediated mechanism of initiation may explain the disparate translation of some eukaryotic messenger RNAs.
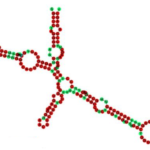 2-D structure of FGF-1 IRES with color-coded sequence conservation. Taken from commons.wikimedia.org and free to use.
2-D structure of FGF-1 IRES with color-coded sequence conservation. Taken from commons.wikimedia.org and free to use.
Fast-forward 30 years, and it has been firmly established that IRES-mediated translation of specific mRNAs in human cells represents an alternative system for synthesis of proteins that are critical for survival, especially under physiological circumstances such as mitosis, apoptosis, hypoxia, and some viral infections when translation of most mRNAs is repressed, as reviewed elsewhere. Importantly, accumulating evidence suggests that malignant cells may be particularly dependent upon IRES-mediated translation, exploiting this mechanism to synthesize certain growth factor receptors, proto-oncogenes, and apoptosis-regulatory proteins to promote their own survival. For example, the fibroblast growth factor 1 (FGF-1) IRES shown here is an RNA element present in the 5' UTR of the mRNA FGF-1 that allows cap-independent translation.
According to Vaklavaset al., approximately 10–15 % of all human genes may be translated via IRESs, and while only a small fraction of these have been identified and characterized, a recent bioinformatics publication concluded that sequence features of viral and human IRESs are predictive of their activity. For all of these reasons, and in view of the increasing interest in IRES-mediated translation, this blog will comment on two emerging applications of IRESs: production of anti-viral vaccines, and investigation of a new class of anti-cancer drugs. In addition, I will also aummarized a recently published novel method for biosensing the integrity of RNA containing IRESs.
IRES-Driven Vaccines
In a Perspective article in Nature Biotechnology by Ulmer and Rappuoli titled Vaccine manufacturing: challenges and solutions, “the prevention of diseases by vaccination is without question one of the most significant medical achievements of mankind. Vaccines currently prevent more than 3 million deaths per year…During the 20th century, the average human life span has increased by approximately 30 years, a significant portion of which has been attributed directly to vaccination.”

The main types of vaccines discussed by Ulmer and Rappuoli include live attenuated viruses that are typically grown in eggs or tissue culture and later purified. In 2008, Volkova et al. reported a new approach, exemplified by IRES-dependent replication of Venezuelan equine encephalitis virus (VEEV). The researchers engineered the VEEV genome to encode for an EMCV IRES element, in order to exclude reversion to the wild-type, pathogenic phenotype. Moreover, this construct would be functional only in cells of vertebrates, and not insects. This genetically modified VEEV demonstrated a highly attenuated phenotype in newborn mice, yet it induced protective immunity against VEEV infection.
As another example of IRES-attenuation, Plante et al. published their investigation of a vaccine for chikungunya fever (CHIK), a mosquito-borne viral disease that causes severe, debilitating, and often chronic joint pain. The chikungunya virus (CHIKV) reemerged from Africa in 2004, and has since afflicted millions of people, including one million people in the Americas since 2013, when it plagued the region for the first time in modern scientific history. CHIKV belongs to the Alphavirus genus in the Togaviridae family.

As an alphavirus, the CHIKV particles shown here are ~50 nm in diameter, and contain a single-stranded, positive-sense RNA genome of 11.8 kb. To develop a safe, rapidly immunogenic vaccine, Plante et al. took advantage of the aforementioned strategy used by Volkova et al., and utilized IRES from EMCV to attenuate alphaviruses. In this method, the IRES is used to replace the sub-genomic promoter for expression of the structural genes through internal initiation on the genomic RNA, greatly reducing structural protein production. Furthermore, the EMCV IRES functions inefficiently in insect cells, thereby preventing infection of mosquito vectors and enhancing safety for use in non-endemic locations.
Plante et al. conducted serial murine brain passages in neonatal mice to demonstrate the stability of the genetic attenuation of CHIKV/IRES, thus “supporting its eligibility for human testing.” This IRES-enabled attenuation strategy for CHIKV has been successfully implemented for eastern equine encephalitis viruses, as well as for the arthritogenic relative of CHIKV, Mayaro virus, both of which are spread to people by infected mosquitos. All of these vaccines have been demonstrated as safe, immunogenic, and efficacious in rodent models, and the CHIK version has been shown as similarly effective in non-human primates.
IRES-Mediated Translation and Cancer

As noted above in the introduction, Vaklavas et al. state that malignant cells may exploit IRES-mediated translation to synthesize certain growth factor receptors, proto-oncogenes, and apoptosis-regulatory proteins in order to promote their own survival. They and others have shown that IRES-mediated translation of oncogenic mRNAs such as c-myc, a pro-oncogene that encodes a transcription factor, and insulin-like growth factor 1 receptor (IGF1R), may be responsible for or contribute to resistance to therapy and enhanced survival of malignant cells under suboptimal microenvironmental conditions, such as those to which tumor cells are exposed in vivo.
Based on earlier research by Vaklavas et al., small molecule inhibitors of IRES-mediated translation were found. Their mechanism of action was confirmed, and effects on the c-myc and IGF1R IRESs were examined in detail. The identification of these compounds allowed for selective perturbation of IRES-mediated translation in its native context, and for the investigation of its relationship to the malignant phenotype. Continuous exposure to the lead compound named “cpd_P” {N-(4-anilinophenyl)-N′-[2-(4-chlorophenyl)ethyl>
thiourea} resulted in cell death.

Triple-negative SUM159 breast tumor cells were treated with increasing concentrations (0–10 μg/ml) of IRES inhibitor cpd_P for periods of 24 to 120 h. Cell survival is assessed using ATP (CellTiter-Glo) as endpoint, presented relative to vehicle (0.1 % DMSO) control (=100 %), plotted on a logarithmic scale. Taken from Vaklavas et al. Tumour Biol. 2016; 37(10): 13247–13264. © The Author(s) 2016. Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided appropriate credit is given to the original author(s) and the source.
Biosensor for IRES Integrity
Because biosensors are trending in nucleic acids, as are IRESs, the combination of these topics seemed to be a strong blog topic, especially in the context of establishing synthetic RNA integrity. This is exactly what a team of researchers at Imperial College London has achieved and published in the journal Biosensors and Bioelectronics this past May, in a paper by Ramirez et al. titled Low-cost and user-friendly biosensor to test the integrity of mRNA molecules suitable for field applications. With the kind cooperation and permission of principal investigators Ignacio Ramirez and Karen Polizzi, pictured here, I was able to obtain and share the graphics in this blog.

By way of an introduction, Ramirez et al. note that RNAs are “cornerstone molecules in biology, relevant in a wide diversity of processes,” with a clear example being the emergence of RNA vaccines. For this reason, there are optimized and commercially available techniques for vaccine detection, characterization, and quantification, ranging from the very simple to the highly complex. According to a recent review, most of the existing RNA biosensors are based on complementary oligonucleotide probes. However, Ramirez et al. conclude that a common characteristic among all existing biosensors is their high technological requirements, which makes them unsuitable for field-based, point-of-care applications and/or non-specialist end-users, such as in many rural locations and underdeveloped countries where vaccine campaigns are critical.
This disconnect between what is available technically vs. what is needed in the field has been addressed by Ramirez et al. in their development of a novel biosensor. Equally important is their proposition that this biosensor would be adequate for more sophisticated and accurate integrity tests, which could in turn be suitable for industrial quality control of RNA vaccines. Next, I will provide a brief synopsis of the work by Ramirez et al., which interested readers can for consult for details.

The key component of the sensor is a bi-functional protein with RNA bio-recognition and bio-reporting properties, namely, a fusion protein between a peptide constituent of the bacterial virus lambda (λ) phage (depicted here), which is referred to as the lambdaN (λN) domain, and the enzyme beta-lactamase (βLac), i.e. λN-βLac. The strong and specific interaction between the λN peptide and the boxB hairpin motif RNA, which λN binds to, has been previously demonstrated: the equilibrium dissociation constant (Kd) is in the range of 1-2 nM. The λN peptide has 22 amino acids, making it is easy to fuse to other proteins and express. The fusion protein λN-βLac, which is designed as the key part of the biosensor, incorporates this peptide as the mRNA recognition element.
As seen in the graphic depiction shown here, the biosensor is based on two binding events that will only take place if the RNA molecule is intact. First, the RNA molecule must be immobilized on poly-deoxythymidine oligonucleotide (orange)-coated beads through its 3’-end poly A tail (yellow). Afterwards, the RNA hairpin binding peptide, i.e. the λN domain from the λN-βLac fusion protein, will specifically recognize its cognate aptamer (the boxB motif; light blue) encoded in the 5’-end of the molecule, thus binding to the immobilized RNA. The β-Lac reporter enzyme (dark blue) portion of the fusion protein can then be used for detection of the molecular integrity of the RNA molecule. This biosensor test can utilize a simple colorimetric assay via a spectrophotometer, or it can be interpreted by visual inspection. As seen here, if RNA degradation occurs, either one or both binding events will not take place (red Xs), and this will block the formation of color.

To fully characterize the system, biolayer interferometry (BLI) analyses were used, since this enabled the study of each step necessary for the biosensor function (in terms of both kinetics and intensity). The results shown here correspond to experiments carried out with the three different model RNA molecules discussed above: a full-length RNA molecule carrying a boxB aptamer in the 5’ end and a polyA tail at the 3’ end; an RNA simulating 5’-degradation, which lacks the nucleotides of the boxB aptamer; and an RNA without both ends, which stimulates a molecule further degraded on its 5’ and 3’ ends.
Biolayer interferometry tests. From top to bottom, binding events as a function of time for three different RNA molecules are assayed: a model full-length RNA molecule with a boxB aptamer (light blue) at the 5’-end and a polyA tail (yellow) at the 3’-end; an RNA lacking the boxB aptamer, thus simulating a RNA degraded from the 5’end; and an RNA without both the aptamer and the polyA tail simulating a RNA further degraded on its 3’ end. A final desorption step is included in the assay. Credit to Ignacio Ramirez and shown with permission.
Ramirez et al. then reproduced the conditions of the BLI assay using a simpler platform based on micrometric magnetic beads and nitrocefin, which is a substrate for β-Lac. Micrometric beads are a good option for the solid support, as they are easy to handle and suitable for miniaturized applications. Likewise, nitrocefin is specifically hydrolyzed by β-Lac with an obvious color change after the reaction, and is therefore a suitable reporter for the assay, because it allows for both visual determination of the reaction, as well as quantitative measurements using spectrophotometry. Interested readers can consult the publication by Ramirez et al. for additional details on how this was achieved.

Biosensor performance measured as spectroscopic response of the biosensor for different percentages of full-length model RNA. The absorbance was measured at 492 nm over time, and was highly linear during the first 10-12 min of assay; a R2 coefficient equal to 0.9761 was obtained from linear fitting of the absorption at 660 s. Credit to Ignacio Ramirez and shown with permission.
For end-user/point-of-care applications where specialized equipment is not available, Ramirez et al. developed a visual test, wherein a sample of beads and nitrocefin reaction mix are loaded onto a hydrophobic surface (e.g. parafilm) with a red background. By a simple visual comparison, it is possible to estimate the amount of full-length RNA: red background dots disappear at distinctly different time points depending on the amount of full-length RNA used in the binding assays. The background dot disappears after 17.3 min for 100 % full-length RNA. Thus, a calibrated background reference will allow for estimation of the integrity of the RNA tested.
Ramirez et al. concluded that the biosensor is reliable enough to be used for analysis of RNA, and it is also capable of detecting the integrity of mRNAs with less than 35 ng of analyte. This amount is comparable to that required for microfluidic electrophoresis, which is the standard RNA quantification and characterization technique. The authors also point out that the commonly used and relatively technically complex Agilent 2100 Bioanalyzer system has a limit of detection above 25 ng.
While Ramirez et al. believe that this is the first reported biosensor to measure mRNA integrity without requiring specialized equipment, it was noted that the mechanism of the sensor requires the incorporation of an aptamer in the 5’-end. Optimization may be required to ensure that the presence of the aptamer in the 5’ UTR is not disruptive for the mRNA function. In addition, there could be non-specific binding between the RNA-binding peptide and the mRNA, the probability of which increases for longer transcripts.
Concluding Comments
Before writing this blog, my knowledge regarding IRESs was limited, and I had very little appreciation for their importance as an alternative pathway for translation of RNA into protein. For this reason, researching the IRES literature was a real learning experience for me. My interest in IRESs was actually prompted by an online abstract of the Ramirez et al. biosensor publication, which piqued my curiosity and led me to contact Dr. Polizzi to ask several technical questions. That article, her answers, and the literature I found led me to writing this blog, first by introducing and exemplifying IRESs, and then by segueing into the Ramirez et al. biosensor paper. Put another way, the events described in the storyline are actually in reverse chronological order from how they occurred!
In any case, I hope you found these topics as interesting as I did, and as always, I welcome your comments.
Addendum
After this blog was written, Jiang et al. published a paper titled Oligonucleotide sequence mapping of large therapeutic mRNAs via parallel ribonuclease digestions and LC-MS/MS. This publication is a prime example of methodology for analysis of mRNA that is at the other end of the spectrum, if you will, relative to the biosensor described in this blog. In brief, Jiang et al. report a novel bottom-up oligonucleotide sequence mapping workflow combining multiple endonucleases. RNase T1, colicin E5, and mazF were applied in parallel to provide complementary sequence coverage for large mRNAs. Combined use of multiple endonucleases resulted in significantly improved sequence coverage: greater than 70% sequence coverage was achieved on mRNAs near 3000 nucleotides long. The workflow is sensitive and specific enough to detect minor sequence impurities, such as single nucleotide polymorphisms (SNPs), with a sensitivity of less than 1%.











