- Oligos Are Not “Magic Bullets”
- Oligos Have, Nevertheless, Enabled New Drug Paradigms
- Oligos Continue to Attract Significant Corporate Investments
Reflections
The 10th Annual Meeting of the Oligonucelotide Therapeutics Society (OTS) is in full swing today in San Diego, CA where it began on Oct 12 and concludes on Oct 15. Having worked on antisense oligos since the early days (~30 years ago) participating in this meeting led me to several thoughts that I wish to share with you in this post.
First of all, contrary to many sceptics in those early days, the concept of using synthetic oligonucleotides as an entirely new class of medicinal agents has not only survived but also greatly expanded in terms of the biological target/mechanism of action and types of oligo constructs used—each with a seemingly endless array of chemical modifications to evaluate. In approximate chronological order of discovery, these targets and the types of oligos they have now come to include are listed below coincidentally, most of these are represented in the 2014 OTS agenda.
mRNA
dsDNA
transcription factors
immunostimulation
proteins
splice junctions
RNA interference
antisense
triplex
dsDNA decoys
CpG
aptamers
blockers
siRNA
Secondly, while it has been possible for oligo chemists to design and synthesize a plethora of modified oligos to achieve optimized nuclease stability, binding to target, etc., efficient delivery has remained the single most challenging problem to deal with. In talks on medicinal oligos, this situation is oftentimes eluded to as something to the effect of “there are only three remaining problems to solve: delivery, delivery, and delivery.”
Lastly, contrary to early hopes of being Dr. Ehrlich’s “magic bullets” (see caption below), oligos didn't quite prove to be the new paradigm for a speedy concept-to-clinic solution. As all of us in the oligo world know, oligo-based therapeutics have encountered long and costly R&D timelines and clinical development paths typical of all other classes of therapeutic compounds.
Dr. Ehrlich's Magic Bullet is a 1940 biographical film starring Edward G. Robinson, based on the true story of the German doctor and scientist Dr. Paul Ehrlich (14 March 1854 – 20 August 1915), who worked in the fields of hematology, immunology, and chemotherapy. His laboratory discovered arsphenamine, a synthetic arsenic-containing compound that provided the first effective medicinal treatment for syphilis, thereby initiating and also naming the concept of chemotherapy. Ehrlich popularized the concept of a "magic bullet". In 1908, he received the Nobel Prize in Physiology or Medicine for his contributions to immunology.
Notable Advances
Given the aforementioned breadth of therapeutic targets being pursued with oligos, selecting notable advances for comment here is necessarily limited to only a few, and is obviously highly subjective. Having said that—and with apologies for not highlighting other important advances—here are my favorites.
Ebola Drug - As reported in a Scientific American story, Thomas W. Geisbert, now at the University of Texas Medical Branch at Galveston, and his many collaborators have devised a highly promising treatment that has saved the lives of six monkeys infected with Ebola virus—the causative agent of the current Ebola disease outbreak recently commented on in a previous post. One of Geisbert's collaborators, Ian MacLachlan of Burnaby, British Columbia–based Tekmira Pharmaceuticals, and his team have received a $140-million grant from the U.S. Department of Defense to develop the therapy further.
Working together, the scientists engineered an siRNA to prevent the Ebola virus from making a particular protein, without which it cannot replicate itself. “If you knock out that one, in theory you knock out everything,” Geisbert says. The researchers also designed another siRNA to thwart manufacture of a second protein that the virus uses to weaken an infected individual's immune system. There is no danger of the siRNAs interfering with typical cellular duties because the targeted viral proteins do not exist in the cells of humans or other mammals.
MacLachlan and his colleagues encapsulated the lab-made siRNAs in Tekmira’s proprietary lipid nanoparticle (LNP) formulation to enable vascular delivery and transport across cellular membranes.
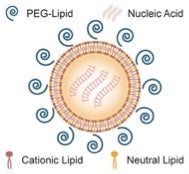 Simplified cartoon of Tekmira’s lipid nanoparticles (LNPs) formulations of siRNA (taken from an interview with Ian MacLachlan in GEN via Bing Images).
Simplified cartoon of Tekmira’s lipid nanoparticles (LNPs) formulations of siRNA (taken from an interview with Ian MacLachlan in GEN via Bing Images).
Then they injected the preparation into several rhesus macaques, which had been infected with Ebola virus less than an hour earlier. In one study, two of three monkeys given a total of four doses of the treatment in the first week after exposure survived. In a second study designed to test the effectiveness of a higher dose, all four monkeys that received seven siRNA injections lived. Tests revealed that the treated monkeys had far fewer virus molecules in their blood than is typical for an infected animal. The macaques tolerated the siRNA injections well, and those that survived were still healthy 30 days later.
The study was a “milestone,” says Gary Kobinger of the University of Manitoba, who is working on a different Ebola treatment based on antibodies. He believes Geisbert and his team “are leading the effort toward clinical development.”
Exon Skipping – This novel strategy is used to restore the reading frame within a gene. Exons are the sections of DNA that contain the instruction set for generating a protein; they are interspersed with non-coding regions called introns. The introns are later removed before the protein is made, leaving only the coding exon regions.
Splicing naturally occurs in pre-mRNA when introns are being removed to form mature-mRNA that consists solely of exons. The mechanism behind exon skipping is a mutation specific antisense oligonucleotide (AON). The AON binds to the mutated exon, so that when the gene is then translated from the mature mRNA, it is “skipped” over, thus restoring the disrupted reading frame. This allows for the generation of an internally deleted, but largely functional protein.
Shown below is a schematic diagram of how this applies to the AON named Drisapersen, developed by Prosensa and currently in late stage development for treating Duchenne Muscular Dystrophy (DMD) patients. Drisapersen induces exon 51 skipping in the dystrophin gene and is intended for ~13% of all DMA patients. On June 3, 2014, Prosensa announced that the United States Food and Drug Administration (FDA) outlined a regulatory path forward for drisapersen, under an accelerated approval pathway, based upon existing data.
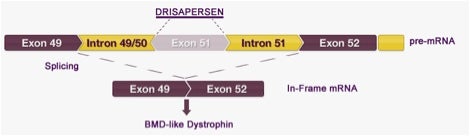 Drisapersen binds to exon 51 in pre-mRNA leading to an in-frame mRNA transcript that produces a shortened but functional protein like that in Becker muscular dystrophy (BMD), which is a less severe form of Duchenne muscular dystrophy.
Drisapersen binds to exon 51 in pre-mRNA leading to an in-frame mRNA transcript that produces a shortened but functional protein like that in Becker muscular dystrophy (BMD), which is a less severe form of Duchenne muscular dystrophy.
Notable Corporate Investments
Santaris Pharma - According to an Aug 4, 2014 press release, Roche announced that it has agreed to acquire Santaris Pharma based near Copenhagen, Denmark. Santaris Pharma has pioneered its proprietary Locked Nucleic Acid (LNA) platform that has contributed to an emerging era of RNA-targeting therapeutics.
Roche plans to maintain Santaris Pharma’s operations in Denmark, where the existing site will be renamed Roche Innovation Center Copenhagen. Under the terms of the agreement, Roche will make an upfront cash payment of $250 million to Santaris Pharma shareholders and make additional contingent payments of up to $200 million based on the achievement of certain predetermined milestones.
Isis Pharmaceuticals - As detailed in multiple press reports here, Isis Pharmaceuticals has announced that it has earned three $1 million payments for advancement of each of three programs with GlaxoSmithKline (GSK), a $10 million milestone payment from Biogen-Idec, and a $15 million milestone payment from AstraZeneca.
Isarna Therapeutics - It was announced earlier this year that Isarna Therapeutics GmbH raised $7.6 million from its current investor groups to further its TGF-ß therapeutics using oligos.
In closing, my reflections turned to thinking that, while it has taken more time and money than originally envisaged by early proponents of medicinal oligos, the pipelines are full and investment is strong.
All of us in the oligo therapeutics arena are greatly interested in hearing about what you are doing with medicinal oligos, so please feel free to share in the comments section below. Also, if you're at OTS stop by the TriLink booth or look for me during the talks, I'd love to meet you face-to-face.
#OligoMeeting2014







