- What is it?
- Why is it Hot?
- How do Nucleic Acids Fit in?
The upcoming Oligonucleotide Therapeutics Society (OTS) annual meeting on Oct 12-15 in San Diego has an agenda with a number of presentations that could each qualify as a hot topic to highlight in this blog post. While mulling over which to pick, the talk by Prof. Weihong Tan—an extraordinarily prolific researcher at the University of Florida—entitled DNA-based molecular medicine and nanomedicine triggered my decision to comment on nanomedicine because it’s at the nexus of multiple scientific disciplines and various aspects of medicine.
What is Nanomedicine?
Nanomedicine, like almost everything these days, has a wiki site; however, it simply defines nanomedicine as “the medical application of nanotechnology,” which begs questions of what’s nanotechnology and what are some of these applications? So, I dug deeper and found—to my surprise—that there is no widely agreed upon definition of nanomedicine!
Instead, several related but different definitions of nanomedicine have been adopted by groups of expert researchers that actually practice nanomedicine. There’s a scholarly editorial in the International Journal of Nanomedicine (IJM) that addresses this curious circumstance. At the risk of “losing you” by being pedantic, here are several snippets that were my key takeaways from this article:
- “Although defining a term such as nanomedicine may sound simple, by comparing several main funding agencies from around the world, one quickly realizes that a uniform international definition of nanomedicine does not currently exist. This is typical of a new field, but can be problematic to those trying to understand the field, make significant contributions to it, and especially in how the public views nanomedicine.”
- “[In NIH’s 2006>
Roadmap for Medical Research in Nanomedicine, [it>
is defined as ‘an offshoot of nanotechnology [that>
refers to highly specific medical interventions at the molecular scale for curing disease or repairing damaged tissues, such as bone, muscle, or nerve’.”
- “[N>
anomedicine emerged from nanotechnology which is generally defined by the creation and use of materials at the level of molecules and atoms (sometimes specifically less than 100 nm, other times this dimension is more diffuse and confusing).”
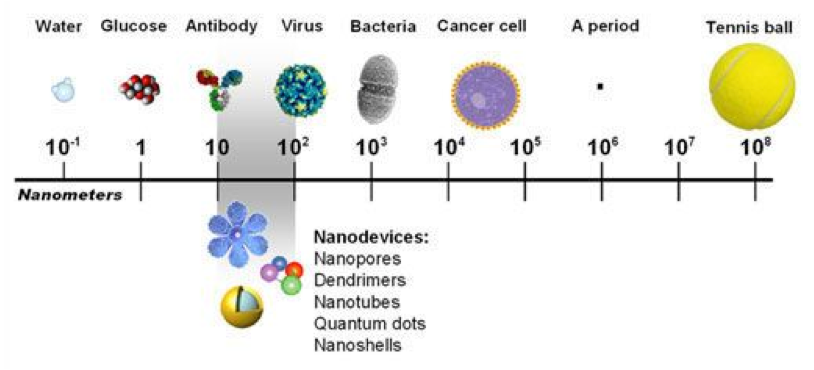
The last point resonated with me, and prompted a search for a picture that puts nanometer dimensions into perspective relative to familiar objects. The scale shown below nicely achieves that and highlights the 10-100 nm range applicable to representative nanodevices used in nanomedicine.
In closing this section, it’s worth pointing to two authoritative sources of up-to-date information on nanomedicine. First is the NIH website dedicated to nanomedicine, and second is the American Society for Nanomedicine (ASN) website. BTW, the current president of ASN is Georgetown University Prof. Esther Chang, whose cell-targeted nanoparticle delivery investigations were featured here earlier this year in a post entitled Nanomedicine: Using ‘Tiny Little FedEx Trucks’ to Target Breast Cancer Tumors.
Why is Nanomedicine Hot?
Before offering my personal perspectives on why nanomedicine is hot, perhaps I should first convince you that it is hot. For instance, entering a general search of PubMed using the word nanomedicine found over 8,000 publications since 1999, with the number of nanomedicine-related publications growing exponentially since 2006—coinciding with the aforementioned NIH Roadmap’s funding. These articles reached an impressive rate of ~4 publications per day in 2012.
This intense interest in nanomedicine over that short period is underscored by a similar search of Google Scholar that found over 97,000 items, while that for Google Patents found nearly 24,000 items.
As for why nanomedicine is hot, let me first echo what I said in the introduction above, i.e., nanomedicine is at the nexus of multiple scientific disciplines and various aspects of medicine. Given this circumstance, nanomedicine as an application is exceptionally diverse and, hence, powerful. This potential power, which can enable major advances having medical significance, is emphasized in the concluding statement taken from the IJM editorial mentioned above:
“IJN takes a firm stance…and emphasizes nanomedicine research in which significantly changed medical events are elucidated only by concentrating on nanoscale events. In this respect, our attempt to separate nanomedicine from other traditional medical research fields is a focus on significantly changed medically related events that result by concentrating solely on the nanoscale.”
How do Nucleic Acids Fit in?
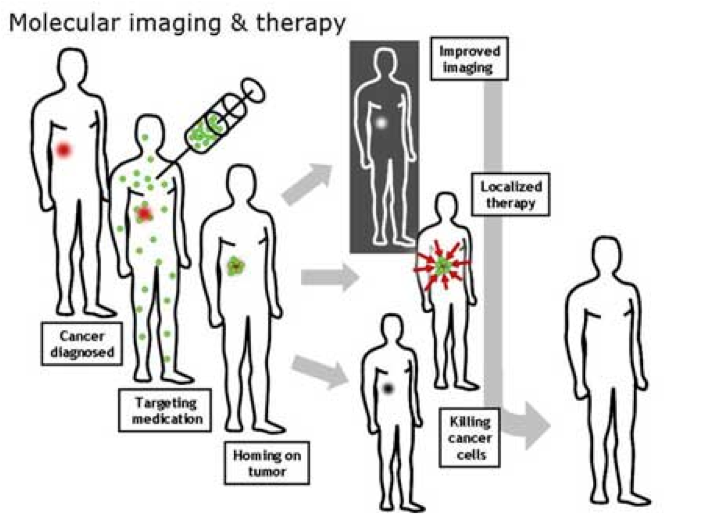
I’m neither clairvoyant nor privy to Prof. Tan’s upcoming lecture at OTS, but I’ve used the illustration below to point out a prime example of the kind of application his talk will likely refer to: tumor-specific, nanoparticle-encapsulated, drug delivery enabled by nucleic acid aptamers. This therapeutic application of aptamers—and its “chemical cousin” wherein imaging agents are delivered—can be read about in two earlier posts from October and November of last year. In brief, nucleic acids provide 3D-structure for aptamers to allow specific bonding to “molecular markers” on target cells or tissue.
More futuristic applications of DNA in nanomedicine are predicated on predictable “self-assembly” of nano-scale structures via base pairing to achieve a functional state. This remarkably powerful attribute is unique to nucleic acids. This is elaborated on in the following abstract of a review by Chhabra et al. entitled DNA Self-assembly for Nanomedicine:
“Self-assembling DNA nanostructures based on rationally designed DNA branch junction molecules have recently led to the construction of patterned supramolecular structures with increased complexities. An intrinsic value of DNA tiles and patterns lies in their utility as molecular pegboard for deterministic positioning of molecules or particles with accurate distance and architectural control. This review will discuss the state-of-art developments in self-assembled DNA nanostructural system. Biomedical aspects of information guided DNA nanostructures will also be summarized. We illustrate both the use of simple DNA artworks for sensing, computation, drug delivery and the application of more complex DNA architectures as scaffolds for the construction of protein and nanoparticle arrays.”
The Future is Now!
You may be thinking that achieving self-assembling DNA nanostructures for practicing nanomedicine is decades away and, even if doable then, it will be limited to only a handful of experts. Parabon NanoLabs, however, is proving that may not be true.
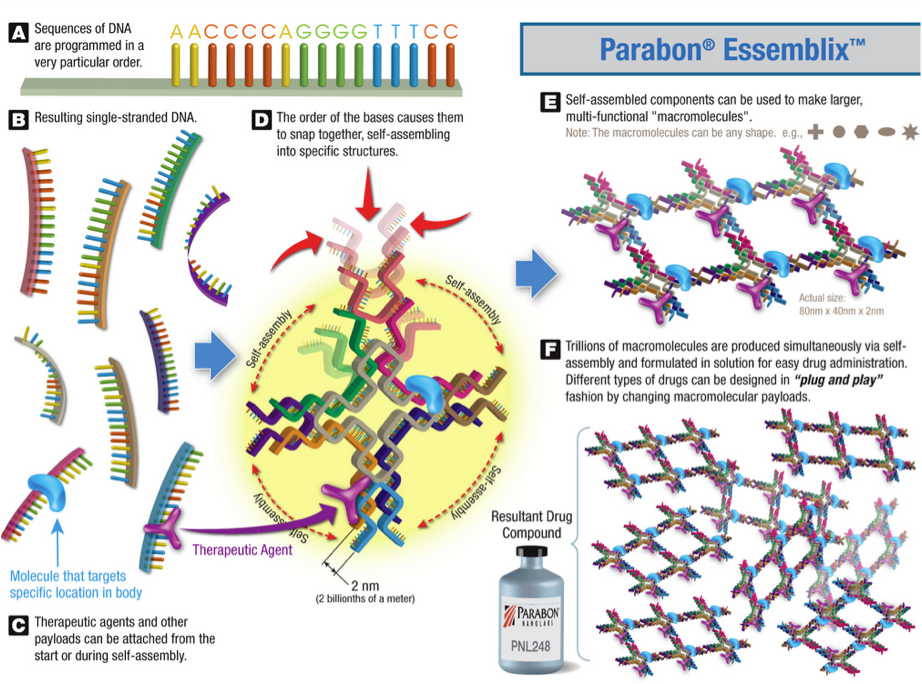
Parabon NanoLabs, with the support of an NSF Technology Enhancement for Commercial Partnerships grant, is using a simple “drag-and-drop” computer interface and DNA self-assembly techniques, to develop a new automated method of drug development that could reduce the time required to create and test medications.
“We can now ‘print,’ molecule by molecule, exactly the compound that we want,” says Steven Armentrout, the principal investigator on the NSF grants and co-developer of Parabon’s technology, according to an online interview. “What differentiates our nanotechnology from others is our ability to rapidly, and precisely, specify the placement of every atom in a compound that we design.”
The Parabon Essemblix Drug Development Platform combines computer-aided design (CAD) software with nanoscale fabrication technology, developed in partnership with Janssen Research & Development, LLC, part of the Janssen Pharmaceutical Companies of Johnson & Johnson.
Scientists can use the CAD software to design molecular pieces with specific, functional components for development of a new drug-delivery agent, as depicted below. The software then optimizes the design using a cloud supercomputing platform that uses proprietary algorithms to search for specific sets of DNA sequences that can self-assemble those components.
With the resulting sequences, the scientists chemically synthesize trillions of identical copies of the designed molecules. The process, from conception to production, can be performed in weeks, or even days — much faster than traditional drug discovery techniques that rely on trial and error for screening potentially useful compounds, the company says.
Parabon R&D scientists also claim that in vivo experiments, funded by an NSF SBIR award, have validated this approach to faster, more targeted rational drug design, which—as the saying goes—is a good thing.
Frankly, I’m amazed and truly excited about this advance in nanomedicine made possible by oligo base-pairing rules! I also look forward to hearing Prof. Tan’s talk at OTS and finding out more about the latest developments in nanotechnology. If you’re attending OTS, please look for me at the talks as I’d really like to meet you!
As always, your comments are welcomed.