“Power of PCR” as a Transformative Diagnostic Method
- FDA Approves Roche PCR Test for Cervical Cancer Screening
- Automated Test Replaces Pap Test as First-line Cervical Cancer Screening
- Demonstrates the “Power of PCR” as a Transformative Diagnostic Method
Pap Test
The Papanicolaou test—aka Pap test, Pap smear, cervical smear, or smear test—is a method of cervical screening used to detect potentially pre-cancerous and cancerous processes in the endocervical canal of the female reproductive system. Unusual findings are often followed up by more sensitive diagnostic procedures, and, if warranted, interventions that aim to prevent progression to cervical cancer.
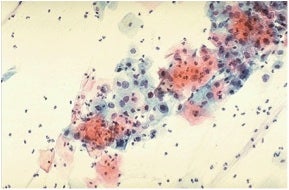
Proper interpretation of microscopic results requires a “trained eye.” This is evident from the representative example shown below, which I found in an online educational textbook and, quite frankly, had trouble discerning the visual keys described in the verbatim caption. Notwithstanding this issue, Pap tests were—until now—the accepted “gold standard.”
The source document for this Pap smear reads as follows. “The cytologic features of normal squamous epithelial cells can be seen at the center top and bottom, with orange to pale blue plate-like squamous cells that have small pyknotic nuclei. The dysplastic cells in the center extending to upper right are smaller overall with darker, more irregular nuclei.”
The eponymous test was pioneered by Georgios Papanikolao, a prominent Greek doctor, who in 1928 was the first to report that uterine cancer could be diagnosed by means of a vaginal smear. However, the importance of this work was not widely recognized until his 1943 publication of Diagnosis of Uterine Cancer by the Vaginal Smear, coauthored by Herbert F. Traut, both at Cornell University Medical College.
 Georgios Papanicolaou moved to Miami, Florida in 1961 to establish the Papanicolaou Cancer Research Institute at the University of Miami, but died in 1962 prior to its opening. Papanicolaou was the recipient of the Albert Lasker Award for Clinical Medical Research in 1950—this award is sometimes referred to as "America's Nobels," as eighty-six Lasker laureates have received the Nobel Prize. Papanikolaou's portrait appeared on the Greek 10,000-drachma banknote of 1995-2001, prior to its replacement by the Euro.
Georgios Papanicolaou moved to Miami, Florida in 1961 to establish the Papanicolaou Cancer Research Institute at the University of Miami, but died in 1962 prior to its opening. Papanicolaou was the recipient of the Albert Lasker Award for Clinical Medical Research in 1950—this award is sometimes referred to as "America's Nobels," as eighty-six Lasker laureates have received the Nobel Prize. Papanikolaou's portrait appeared on the Greek 10,000-drachma banknote of 1995-2001, prior to its replacement by the Euro.
Cervical Cancer Statistics
Cervical cancer is the second most common cancer in women worldwide, according to an NIH publication in 2007. Country-by-country data for cervical cancer reveal a striking geographical distribution. According to currently available U.S. Centers for Disease Control (CDC) FastStats, cervical cancer mortality in the U.S. in 2010 was ~4,000 or ~2.5 deaths per 100,000 females.
The global statistics provided by Cancer Research U.K. are far more saddening. Worldwide there were more than ~275,000 deaths from cervical cancer in 2010 that accounted for ~10% of female cancer deaths.
Remarkably, mortality rates are reported to vary seventeen-fold between the different regions of the world. By estimating the years-of-life-lost (YLL) by young and middle-aged women (25-64 years old) in different regions of the world, YLL attributed to cervical cancer is the most important cause of YLL for all cancers in Latin America, the Caribbean, and populous regions of Sub-Saharan Africa and South-Central Asia. The overall picture is not very sensitive to the age-weighting function used. The report notes that, since this loss of life is preventable using existing technologies, more health-resource allocation in low income settings is needed.
Pap Test Statistics
Currently available CDC FastStats for Pap test use in the U.S. in 2010 (the most recent year available) are as follows:
- Percent of women 18 years of age and over who had a Pap test within the past 3 years: 73.2%
- Number of physician office visits during which Pap tests were ordered or provided: 29.4 million
- Number of hospital outpatient department visits during which Pap tests were ordered or provided: 2.4 million
Pap Test Recommendations as of 2013
To put today’s blog-post headline about switching from Pap to PCR in perspective, here are snippets from the most recent CDC guidelines and comments made available in a January 2013 press release headlined with “more women getting Pap tests as recommended [but>
some women get Pap tests without need.”
- In 2012, the U.S. Preventive Services Task Force, American College of Obstetricians and Gynecologists and American Cancer Society recommended that women, beginning at age 21, should start Pap test screening every three years.
- The same groups agree that screening is unnecessary for most women who have had a total hysterectomy (removal of the uterus and uterine cervix) for non-cancerous reasons, or for women aged 65 years and older with several years of normal test results.
- Studies analyzed Pap test survey data from CDC’s Behavior Risk Factor Surveillance System found the following:
- The percentage of women aged 18-21 years who reported never being screened increased from 23.6% in 2000 to 47.5% in 2010; however, screening is not recommended for women under the age of 21.
- In 2010, 58.7% of women aged 30 years and older who had a hysterectomy were still given a Pap test.
- Because of the Affordable Care Act (aka Obamacare), many private health plans and Medicare now cover certain preventive services, including cervical cancer screening, with no copays or other out-of-pocket costs.
HPV: The Cervical Cancer-Causing Agent and Key to Early Detection
In a landmark publication in 1999 entitled Human papillomavirus is a necessary cause of invasive cervical cancer worldwide, Dutch investigators used PCR data to establish that the worldwide HPV prevalence in cervical carcinomas is 99.7 per cent. They noted that “the presence of HPV in virtually all cervical cancers implies the highest worldwide attributable fraction so far reported for a specific cause of any major human cancer.” More importantly, they presciently concluded that “the extreme rarity of HPV-negative cancers reinforces the rationale for HPV testing in addition to, or even instead of, cervical cytology in routine cervical screening.”
Due in part to technical challenges posed by numerous genotypes of HPV with varying cancer causality detailed elsewhere, and unavoidable time-consuming clinical studies required for FDA approval, it has taken ~15 years for a PCR test to now be poised to displace the Pap test as the primary diagnostic approach for early detection of cervical cancer.
Those of you who are interested in the technical underpinnings of Roche’s investigations to this end are referred to this 2013 publication by Roche and collaborators entitled Development and characterization of the cobas human papillomavirus test. In contrast to the tedious Pap test protocol and its “visually challenging” manual microscopic analysis, this “cobas”-based PCR test provided by Roche is fully automated. The test process involves two instruments: one that completes sample preparation (COBAS® AmpliPrep) and another that performs the PCR process and detection of the pathogen DNA in real time (COBAS® TaqMan® Analyzer).
Incidentally, I traced-back the term “cobas” to late 1970’s Roche instrumentation named the “cobas-bio” analyzer, but could not decipher what “cobas” stands for! If any of you know the answer, please let us know by a comment at the end of this post.
FDA Panel Recommends Replacement for the Pap Test
This attention-grabbing headline of a March 2014 NY Times article by Andrew Pollock was the catalyst for my decision to research and write this blog exemplifying the “power of PCR” as a transformative diagnostic method. While this and numerous other popular news media all made reference to an FDA panel’s report, it took some digging to find the actual source-report, which is an 80-page pdf that can be accessed here to peruse in detail, if you wish. However, a much shorter but essential-fact-laden article by Joyce Frieden, News Editor of MedPage Today provided the following excerpts.
The FDA's Medical Devices Advisory Committee Microbiology Panel agreed by a vote of 13-0 in each of three successive votes that the cobas® viral DNA test for HPV—made by Roche Molecular Systems—was safe and effective for cervical cancer screening, and that the benefits of the tests outweighed the risks. The Panel recommended that this Roche HPV test replace the Pap smear as the first-line standard of care for cancer screening.
The Roche test is seen as better than Pap tests in finding precancerous lesions (taken from the NY Times)
The cobas® test currently has approval as a follow-up assessment for women 21 and older who have abnormal Pap tests, and as a co-test with the Pap smear to screen for the high-risk p16 and p18 HPV strains in women 30 to 65. The test comprises genotyping for HPV16 and 18 and pooled assessment of 12 additional high-risk HPV strains.
According to the proposal submitted by Roche, women 25 and older who test positive for HPV16 or 18 would proceed directly to colposcopy for further assessment.
Patients who test negative for HPV16 or 18 but positive for the other high-risk strains would have a Pap test to determine the need for colposcopy. Women who have a completely negative test would be followed at their physician's discretion.
Panelists did express some concerns about dropping the age at which women should have the test from 30 to 25. The ATHENA study of over 47,000 patients with long-term follow-up used as the basis for the application found that about 11% of women ages 25 to 29 tested positive for HPV16 or 18 with the cobas test, compared with 7.28% among women 25 to 29 who had cytology alone as their first-line screening. Panel member Paula Hillard, MD, of Stanford University in California, was quoted as saying that would mean more patients in that age group "will be anxious about potentially having cancer."
In addition, Hillard is quoted as expressing concern about off-label use. "I'm concerned that all those women potentially with other high-risk positivity won't go to Paps next but go [straight>
to colposcopy. That's not what's proposed here, but what control does FDA have once it's out there?"
Panelist Kenneth Noller, MD, of the American Board of Obstetrics and Gynecology, in Dallas, agreed that real-world use could differ from the protocol proposed by Roche. He’s quoted as saying that "I've been watching how people practice; if you're high-risk HPV positive you're going to get colposcopy." Furthermore, he said "that doesn't necessarily mean it's bad—it’s what you do with the colposcopy."
Noller added that although he was "somewhat biased against dropping the age to 25 before I came here ... I find the data presented today somewhat compelling to drop it to 25."
Agreeing with this was panel member Kimberly Hanson, MD, MHS, of the University of Utah and ARUP Laboratories, both in Salt Lake City: "now we have the opportunity to identify women earlier, and to me that's compelling," adding that "although colposcopy is invasive and can be anxiety-provoking, it's really very safe, so I think I'm leaning toward earlier screening."
According to the summary submitted by FDA staff members, "The data show that the proposed primary screening indication for the cobas HPV test detects more women with disease and requires fewer women without disease to go to colposcopy than cytology alone."
Benefit-risk analyses favored the HPV DNA test whether expressed in terms of number of cases of high-grade cervical disease per 10,000 women screened or per 100 colposcopy procedures.
The FDA is not bound to follow its advisory committees' recommendations, but does so in most cases. On April 25—coincidentally DNA Day 2014—the FDA formally approved Roche’s HPV test as the First-Line Cervical Cancer Screening Method.
The “Entrenchment Factor”
At the risk of “throwing cold water” on the aforementioned PCR test benefits, I feel compelled to quote from Pollak’s NY Times story that ended with the following caveat.
“The Pap test, which is well entrenched and has been highly successful, will not go away quickly, if at all, however.
Assuming the FDA itself agrees with its advisory committee and approves the new use of Roche’s test, it would become just another option, not a replacement for the older testing regimens. And many doctors will not adopt the new test unless professional societies recommend it in guidelines, which could take years.”
Let’s all hope that these professional societies—and any other persuasive factors—lead to relatively rapid adoption by doctors.
As always, your comments are welcomed.