- Various RT-PCR Assays for Zika Virus Have now Received Emergency Use Authorization (EUA) from the FDA
- Quest Diagnostics’ Assay Approved by FDA for General Use as a Zika Test
- Troubled Theranos Touts New “miniLab” for Zika EUA
- Vaccine Development Progressing Albeit Relatively Slowly
 Aedes aegypti mosquito. Taken from wcvb.com
Aedes aegypti mosquito. Taken from wcvb.com
In January 2016, I posted a blog about the then emerging public awareness of Zika virus (ZIKV), which is spread by the bite of infected mosquitos—primarily the Aedes aegypti mosquito. Sadly, ZIKV can be passed from a ZIKV-infected pregnant woman to her fetus leading to development of a brain defect (microcephaly) and/or other malformities. Moreover, ZIKV is now associated with sexual transmission and blood transfusion. This is scary news.
This update was prompted by the additional fact that ZIKV is on the rise and there is still no vaccine, according to the Centers for Disease Control and Prevention (CDC)—my “go to” source for loads of authoritative information about this infectious disease. Weekly updated ZIKV-infection “case counts” in US States and US Territories were given as ~1,200 and ~6,500, respectively on Aug 10th, and increasing to ~3,400 and ~20,000 on Sep 21st —only 6 weeks later!
As I pointed out in my previous blog on ZIKV, absent an anti-ZIKV vaccine, there is considerable interest in mosquito abatement as well as early detection, notably by reverse-transcription PCR (RT-PCR) of this RNA Flavivirus. Now that Zika infection by mosquitos in Florida has been found, leading to several CDC warnings, I thought it would be both apropos and technically interesting to provide the following update on ZIKV structure and RT-PCR.
ZIKV Genome and Structure
As of last month, my PubMed search of “Zika [Title/Abstract>
AND RT-PCR [Anywhere>
” gave 55 publications. Incidentally, there are now a number of Zika genome sequencing publications, such as this lead reference. It’s worth noting that ZIKV genome sequencing enables monitoring the potential evolution of new genomic variants that might foil existing RT-PCR assays and guide the selection of new primers for RT-PCR.
 ZIKV genome. Taken from viralzone.expasy.org with permission from SIB Swiss Institute of Bioinformatics, ViralZone
ZIKV genome. Taken from viralzone.expasy.org with permission from SIB Swiss Institute of Bioinformatics, ViralZone
It’s also worth pointing out that Zika’s overall molecular capsid structure is enveloped, spherical, and has a diameter of ~40 nm in diameter, as depicted below in schematic form, and pictured in the accompanying electron microscopic image. The surface proteins are arranged in an icosahedral-like symmetry.
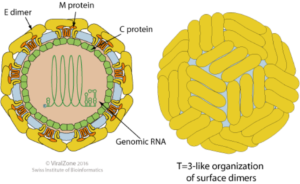 ZIKV capsid structure. Taken from viralzone.expasy.org with permission from SIB Swiss Institute of Bioinformatics, ViralZone
ZIKV capsid structure. Taken from viralzone.expasy.org with permission from SIB Swiss Institute of Bioinformatics, ViralZone

ZIKV RT-PCR
In the interest of giving explicit credit to various investigative groups who have developed RT-PCR assays for ZIKV, here are links and short snippets I’ve selected from publications, in chronological order, found in the aforementioned PubMed search. Interested readers are encouraged to check out the original publication for details.
The first report of an RT-PCR assay for ZIKV appears to be by Faye et al. in 2008 at the Institut Pasteur de Dakar in Senegal, who targeted the envelope protein coding region and tested ZIKV isolates previously collected over a 40-year period from various African countries and hosts. The assay's detection limit and repeatability were respectively 7.7pfu/reaction and 100% in serum; none of 19 other Flaviviruses tested were detected. Faye et al. in 2013 extended this work to include quantitative RT-PCR detection of ZIKV and evaluation with samples from field-caught mosquitoes.
Kinetics of ZIKV detection in urine compared to serum from 6 patients was described by Gourinat et al. in 2015 at the Institut Pasteur, Noumea, New Caledonia using RT-PCR primers and probes previously reported by others. Urine samples were positive for ZIKV more than 10 days after onset of disease, which was a notably longer period than 2-3 days for serum samples. These researchers concluded that urine samples are useful for diagnosis of ZIKV infections, and are preferred to serum wherein virus titer diminishes more rapidly.
Musso et al. in 2015 working in Tahiti, French Polynesia with 1,067 samples collected from 855 patients presenting symptoms of Zika fever found that analysis of saliva samples increased the rate of detection of ZIKV at the acute phase of the disease compared to serum samples. They noted that saliva was of particular interest when blood was difficult to collect, especially for children and neonates.
Most recently, Xu et al. in 2016 in China reported the development of a SYBR Green (intercalator dye)-based qRT-PCR assay for detection of ZIKV. Although their results indicate that the assay is specific, it’s important to note that SYBR-type detection can be subject to nonspecific artifacts, for which TriLink’s proprietary CleanAmp™ Primers can be investigated to potentially ameliorate such problems, as discussed in this downloadable pdf publication by TriLink researchers.
ZIKV In Vitro Diagnostic Assays
As detailed elsewhere, the Secretary of Health and Human Services (HHS) earlier this year determined that “there is a significant potential for a public health emergency that has a significant potential to affect national security or the health and security of United States citizens living abroad and that involves Zika virus.” The Secretary of HHS further declared that “circumstances exist justifying the authorization of the emergency use of in vitro diagnostics for detection of Zika virus…”.
The following RNA-based assays and suppliers are currently listed by the FDA for this Emergency Use Authorization (EUA):
- xMAP® MultiFLEX™ Zika RNA Assay (Luminex Corporation)
- VERSANT® Zika RNA 1.0 Assay (kPCR) Kit (Siemens Healthcare Diagnostics Inc.)
- Zika Virus Real-time RT-PCR Test (Viracor-IBT Laboratories, Inc.)
- Aptima® Zika Virus Assay (Hologic, Inc.)
- RealStar® Zika Virus RT-PCR Kit U.S. (Altona Diagnostics)
- Zika Virus RNA Qualitative Real-Time RT-PCR (Focus Diagnostics)
- Trioplex Real-time RT-PCR Assay (CDC)
Among these, two are particularly notable—in my opinion. Trioplex Real-time RT-PCR Assay is a multiplexed laboratory test developed by the CDC to simultaneously detect ZIKA, dengue virus, and chikungunya virus RNA, each of which can be transmitted primarily by Aedes aegypti mosquitos to cause infections with similar symptoms. Consequently, it is useful to have a single test that can detect each of these viruses in the same sample. Full details for the Trioplex assay are provided in a 40-page downloadable pdf from the CDC at this link.
In brief, this CDC-developed test uses virus-specific primer pairs and fluorogenic hydrolysis probes each differentially dual-labeled with fluorescent reporter and quencher dyes for in vitro detection of complementary DNA (cDNA). The detection follows reverse-transcription of RNA isolated from clinical specimens including serum, cerebral spinal fluid, urine, and amniotic fluid. It’s worth pointing out that I subsequently found a publication in 2016 by several collaborating academic groups regarding an analogous triplex RT-PCR assay for the same three viruses.
The other notable assay is Zika Virus RNA Qualitative Real-Time RT-PCR developed by Focus Diagnostics, which in April 2016 was the first of the aforementioned ZIKV tests to receive authorization by the FDA for use by qualified labs to detect ZIKV RNA in blood samples of those meeting CDC clinical criteria or of people who may have lived in or traveled to an affected location or had other exposure to the virus. Quest Diagnostics, the parent company of Focus Diagnostics, announced it would make the test broadly available to physicians, including those in Puerto Rico in May 2016.
ZIKV EUA Sought by Theranos for Its new “miniLab”
Theranos, which has been in the news regarding troubles over its stealthy proprietary system for finger-stick blood tests, appears to be pivoting its strategic plans. It announced at the August 2016 American Association of Clinical Chemistry Meeting its R&D for a new, fully automated miniLab system, including analytical and method comparison results of its ZIKV nucleic acid-amplification-based assay.
According to its press release, the company collected finger-stick samples from subjects, including people in the ZIKV-infested Dominican Republic, and shipped those to Palo Alto, California to run on the miniLab. Although I was unable to obtain these particular results, I did find the following figure at the TechCrunch website showing functional components of the miniLab along with an article by Sarah Buhr that’s worth a quick read, in my opinion.

According to the aforementioned Theranos press release, the company has submitted assay validation data for this Zika assay to the FDA for an EUA. The company also states that it is unaware of any currently available capillary (i.e. finger-stick) test for ZIKV.
I’ll stay tuned for future general information about the miniLab, as well as information specifically related to ZIKV. If I hear of anything, I’ll add as a comment here or in a new post with technical details about its nucleic acid assays.
ZIKV Vaccine Status
I hope this blog has convinced you that RT-PCR of ZIKV has provided improved molecular diagnostics. I’m guessing, that like me, you find it unfortunate that there isn’t a proven anti-ZIKA vaccine as of yet. This is especially frustrating given the fact that Zika disease has been known for more than 50 years, and that it is evidently on the rise globally, including in the USA according to regularly updated CDC statistics.
In February 2016, the Obama administration requested $1.9 billion in funding for the NIH to develop a ZIKV vaccine. The US Congress continues to be deadlocked by partisan politics despite the fact that Florida state and local officials are scrambling to contain the ZIKV outbreak in Miami Beach. This outbreak poses a serious threat to the health of residents, as well to visitors who drive the region’s $24 billion-a-year tourism industry.
Nevertheless, some progress has been reported for US studies in monkeys, and US-based Inovio says it’s received FDA approval to begin studies in humans. Outside the US, Sanofi Pasteur in France is said to be poised for initiating its trials in humans, and French biotech company Valneva is reported to have succeeded in generating a ‘highly purified inactivated vaccine candidate’ using the same technical approach it used for its encephalitis vaccine that is marketed in the United States and Europe.
Lagging—dare I say “glacially slow”—action against ZIKV by the World Health Organization is quite disappointing to me, and is reminiscent of what I’ve commented on in an earlier blog concerning this bureaucracy’s ineffective response to the Ebola virus. If I had my druthers, TriLink’s previously announced engagement by Battelle in development of Ebola mRNA vaccine would somehow materialize for a Zika mRNA vaccine. In this regard, GlaxoSmithKline is reported to be preparing research studies alongside the NIH's Vaccine Research Center to test a self-amplifying mRNA vaccine technology for Zika. Interested readers can check out this link to a fascinating PNAS publication by Geall et al. on biosynthetic self-amplifying mRNA vaccines delivered in lipid nanoparticles.
As always, your comments are welcomed.
Postscript

One of my previous posts featured recent elucidation of biologically functional G-quadraplexes in living cells. Consequently, it’s apropos to mention here that Fleming et al. at the University of Utah have just now published the first analysis of potential G-quadruplex sequences (PQS) in the RNA genome of ZIKV. As depicted in this artistic cartoon, several PQS were found, with the most stable located near the end of the 3’ untranslated region (3′-UTR). Importantly, these investigators propose a rationale for screening G-quadruplex-binding compounds as a completely new class of anti-ZIKV drug candidates. In my opinion, this is a great example of how basic biochemical research can lead to new strategies for much needed antiviral drugs.






