- Leonard Zon Uses Zebrafish to “Fish” for Candidate Drug Compounds
- Two Candidate Drugs are in Clinical Trials for Cancer Treatments
- Zon Interviews L. Zon
 Leonard I. Zon, M.D. Taken from pediatrics.mc.vanderbilt.edu.
Leonard I. Zon, M.D. Taken from pediatrics.mc.vanderbilt.edu.
The first time I was asked if I was related to the scientist Leonard Zon, I honestly had to reply that I didn’t know, and out of curiosity later looked up his publications, which were quite numerous for a then newish investigator. His current biosketch expertise includes pioneering research in the new fields of stem cell biology and cancer genetics. Dr. Leonard I. Zon is the Grousbeck Professor of Pediatric Medicine at Harvard Medical School, an Investigator with the Howard Hughes Medical Institute, and Director of the Stem Cell Program at Children's Hospital Boston—that’s impressive!
I found Leonard Zon’s unusual zebrafish-based research and accomplishments therefrom definitely blogworthy, and the coincidences of both our surnames and involvement in science are kind of an unusual “double-doppelgänger,” if you will. In any case, it’s always a surprise to meet your double, even if only in name and profession.
A few regular readers of my blog will likely smile and think that Zon on Zon’s Zebrafish is yet another instance of my penchant for alliteration. However, reading the following snippets about Leonard Zon’s clever—dare I say zany—use of zebrafish for his research will illustrate why they are unusual, interesting and commercially viable.
On the other hand, I must admit that I too smiled at the thought of this unique opportunity to post an interview of one Zon by another Zon, which reminded me a bit of Zappa Plays Zappa. But I digress…
Zebrafish as a Model for Organogenesis
Leonard Zon currently has nearly 250 research publications listed in PubMed, which is a large number by any measure, but that’s even more impressive when you take into account that his first was in 2002—only 14 years ago. That translates to an average of about 3 publications every 2 months each year!
Zon’s inaugural publication in 2002 was an in-depth review in venerable Science in which the abstract presciently reads in part as follows:
Organs are specialized tissues used for enhanced physiology and environmental adaptation. The cells of the embryo are genetically programmed to establish organ form and function through conserved developmental modules. The zebrafish is a powerful model system that is poised to contribute to our basic understanding of vertebrate organogenesis. This review develops the theme of modules and illustrates how zebrafish have been particularly useful for understanding heart and blood formation.
As will be elaborated below, Zon’s most recent publication in 2016—also in Science—has extended the zebrafish model to now include melanoma. If you’re asking yourself, why use zebrafish, the answer is partly due to convenience derived from the unique features and accelerated life cycle of zebrafish.
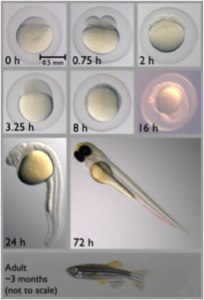 Zebrafish life cycle. Taken from en.wikipedia.com.
Zebrafish life cycle. Taken from en.wikipedia.com.
Seen right, these advantages include its small size, easy care, and rapid generation time. In addition—and very importantly—the embryos and growing zebrafish are transparent, allowing for continuous observation of developing organs under the light microscope.
Mutagenesis screens allow examination of defects in early organogenesis and late organ function. These many advantages of investigating zebrafish—and Zon’s huge facility comprising thousands of tanks—are nicely explained and shown in a video, which I found well worth viewing. The video also includes Zon’s specially bred, virtually transparent species of zebrafish named—humorously—Casper, after Casper the Friendly Ghost. Pictures below are (a) the transparent Casper zebrafish; (b) the non-transparent wild-type zebrafish; and transparent Casper the Friendly Ghost, which brings back my childhood memories. But again I digress…let’s get back to science!
 (a) Casper, (b) wild type zebrafish. Taken from nature.com. Transparent Casper the Friendly Ghost. Taken from enchantedamerica.wordpress.com
(a) Casper, (b) wild type zebrafish. Taken from nature.com. Transparent Casper the Friendly Ghost. Taken from enchantedamerica.wordpress.com
Aided by the availability of DNA sequence information for the zebrafish genome, researchers have published ~2,000 (!) reports dealing with antisense gene “knockdown” using phosphorodiamidate oligonucleotides. By numerical coincidence, the first such report appeared in 2000, and was prophetically entitled Effective targeted gene 'knockdown' in zebrafish.

As shown below, these rather unusual oligonucleotides—dubbed “morpholinos” by resemblance of the 6-membered ring to morpholine—can be injected directly into zebrafish embryos. Interested readers can consult a detailed “how to” guide on use of morpholinos in zebrafish.
Going forward, however, I expect that uber-hot CRSPR/Cas9 gene editing will be widely adopted, based on the titles of these two pioneering publications in 2016:
Stem Cells and Beyond
Now that we know a bit about Zon’s zebrafish and how to knockdown or edit a gene for functional genomics (aka “gene functionation”), let’s zero in on what Zon studies and how he does that. In a nutshell, the overarching science in Zon’s lab deals with stem cells, which are undifferentiated cells that can differentiate into specialized cells, as well as divide to produce more stem cells, as depicted below.

According to Zon’s website, the hematopoietic system that forms various types of blood cells is an excellent model for understanding tissue stem cells. This conceptual relationship is very important because it provides insight to cell differentiation regulation, and involvement in aging, disease, and oncogenesis. In addition, better understandings of the regulation of hematopoietic stem cell biology and lineage differentiation improves diagnosis and treatment of human hematopoietic disorders (aka “blood cancers”) and bone marrow transplantation therapies.

From Zebrafish to Clinic
Scientific theory is nice, but success is best, and Leonard Zon’s theory of using zebrafish to manipulate human stem cells for discovering therapies seems indeed to be headed for success, according to an article in the Harvard Gazette.
Zon and others at the Harvard Stem Cell Institute (HSCI) have published initial results of a Phase Ib safety study wherein 12 adult patients undergoing umbilical cord blood transplantation received two umbilical cord blood units, one untreated and the other treated with the small molecule 16,16-dimethyl prostaglandin E2 (dmPGE2). This molecule had been found in Zon’s zebrafish screen, which I’ll outline below.
Fate Therapeutics, a San Diego-based biopharmaceutical company of which Zon is a co-founder, sponsored the investigational new drug (IND) application, under which the aforementioned clinical program was conducted, thus translating his research findings from the laboratory—dare I say tank—into the clinic.
Zon’s Zebrafish Yield New Approaches to Treat Muscular Dystrophies
According to NIH’s National Institute of Neurological Disorders and Stroke website, muscular dystrophies (MD) are a group of more than 30 genetic diseases characterized by progressive weakness and degeneration of the skeletal muscles that control movement. It adds that there is no specific treatment to stop or reverse any form of MD. Consequently, MD is a compelling target for new drug discovery, and Zon’s zebrafish are being used for such discovery in the following way.
Each zebrafish produces about 200 (!) eggs per week, so Zon’s lab collects and deposits a single egg into each well of a multi-well plate for individual but parallelized treatment with individual chemicals to screen for effects on differentiation, thus screening many compounds in a speedy manner.
Zon and coworkers concluded that “these studies reveal functionally conserved pathways regulating myogenesis across species [zebrafish, mouse, and human> and identify chemical compounds that…differentiate human iPSCs into engraftable muscle.”
Let’s hope that human clinical trials using this novel therapeutic approach enabled by Zon’s zebrafish soon prove successful for treatment of MD.
Zon’s Zebrafish Also Enable Elucidation of Melanoma Development
According to a fact page by the American Cancer Society, skin cancer is the most common of all cancers. About 3.5 million cases of basal and squamous cell skin cancer are diagnosed in this country each year. Melanoma, a more dangerous type of skin cancer, will account for more than 73,000 cases of skin cancer in 2015.
Zon and a large group of 18 coworkers have recently reported in venerable Science work that has been heralded in a New York Times article. This study is very comprehensive and involves lots of “heavy duty” molecular and cellular biology, which you can read in detail in the aforementioned linked article. Snippets of the key findings are as follows.
- Benign melanocytic skin cells carry oncogenic BRAF-V600E mutations and can be considered a “cancerized” field of melanocytes, but they rarely convert to melanoma.
- In an effort to define events that initiate cancer, they used a melanoma model in the zebrafish in which the human BRAF-V600E oncogene is driven by the melanocyte-specific mitfa
- When bred into a p53 mutant background, these fish develop melanoma tumors over the course of many months.
- The zebrafish crestin gene is expressed embryonically in neural crest progenitors (NCPs) and is specifically reexpressed only in melanoma tumors, making it an ideal candidate for tracking melanoma from initiation onward.
- As show below, they developed a crestin:EGFP reporter that recapitulates the embryonic neural crest expression pattern of crestin and its expression in melanoma tumors.
- They show through live imaging of transgenic zebrafish crestin reporters that within a cancerized field (BRAFV600E-mutant; p53-deficient), a single melanocyte reactivates the NCP state, and this establishes that a fate change occurs at melanoma initiation in this model.

Zon Interviews L. Zon

After researching Leonard Zon’s aforementioned unique—and promising—use of zebrafish to advance basic science and discover new therapies, I contacted him by email to “interview” him, regarding several points. My questions (JZ) and his answers (LZ) are as follows.
JZ: Aside from the cord blood clinical trial mentioned in the Harvard Gazette, are any other human clinical trials being carried out based on your zebrafish findings?
LZ: We have had two chemicals discovered in zebrafish, and ultimately went to a clinical trial. The first was a di-methyl form of PGE2 for cord blood transplantation for leukemia. The second was leflunomide, an arthritis drug that paused transcription in neural crest cells and is being evaluated for metastatic melanoma.
JZ: Is Fate Therapeutics your only startup company?
LZ: I started Scholar Rock about 3 years ago. This company is targeting the TGF-B family of ligands. Has about 25 employees, and is in Cambridge. I am about to start a third company.
JZ: Is your zebrafish method for screening chemicals patented?
LZ: We have patents on several screening methods, but in general we patent the chemicals we find.
JZ: Zebrafish offer many reported advantages for your kind of research, but what is a primary disadvantage?
LZ: The major disadvantage of the zebrafish is that the system occasionally lacks definition. For instance, in the blood system, we have one monoclonal antibody against one epitope. We really need to create reagents for the field that brings it in line with other systems such as mice and humans.
JZ: How many tanks and zebrafish are maintained in your two labs?
LZ: We have 4000 tanks and about 300,000 fish.
JZ: Did you name your transparent Casper zebrafish after Casper the Friendly Ghost?
LZ: Absolutely.
In closing, I should add that the huge amount of information on zebrafish as a model organism for human disease and drug discovery from many labs has been centralized and organized in a database that is available through The Zebrafish Information Network (ZFIN) for researchers to share at the ZFIN Community Wiki.
I hope that you found this blog interesting, and I welcome your comments.

Postscript
Truth be told, compared to being asked if I’m related to Leonard Zon, I’m more frequently asked if I’m related to Zon guitars, which apparently are quite well known, and are produced in Redwood City, CA by Joe Zon, who is pictured below. My reply to that frequent question is that I don’t know if I’m related, but will someday look into that, as well as whether I’m distantly related to Leonard Zon.






