mRNA CDMO services
Our streamlined services set you up for success
Leverage a legacy of trailblazing innovation in your mRNA research and discovery with TriLink. Our dedicated staff has specialized mRNA expertise to support your journey, helping you make discoveries, scale up production, overcome regulatory challenges, and get to market faster. Set your organization up for success by partnering with a global leader in mRNA development and manufacturing—one who can help you take a targeted approach to discovering the next life-changing therapeutic.

Design your mRNA or long RNA transcript using our mRNAbuilder™
mRNA CDMO Services to accelerate drug development
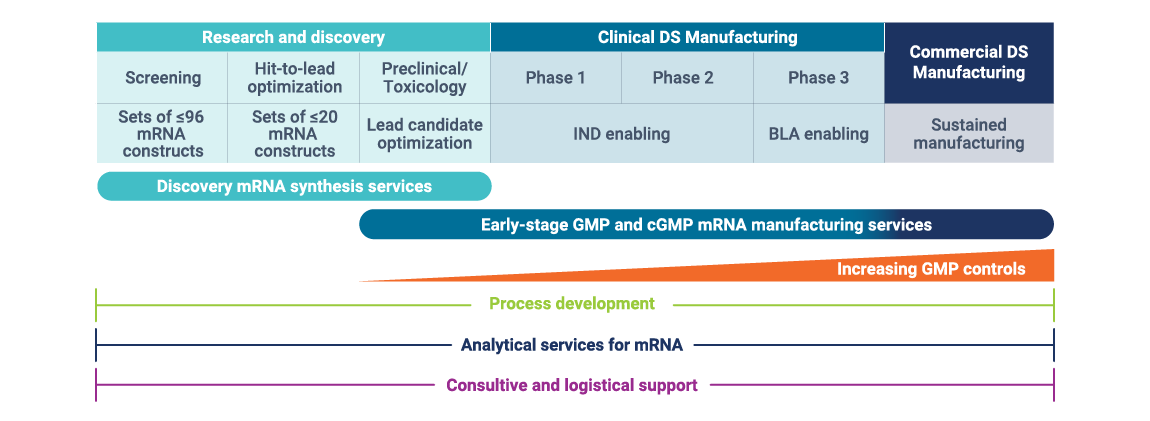
mRNA expertise for each stage of the process

DNA template manufacturing
To shorten timelines and simplify supply chains, our TriLink team can help you integrate phase-appropriate DNA templates into your mRNA manufacturing process

GMP mRNA manufacturing
Bringing your mRNA vaccine or therapeutic through the clinic introduces new demands for process development, scalability, and quality. TriLink’s new cGMP facility, opening in early 2024, has been designed by mRNA and regulatory experts to streamline late-phase clinical mRNA drug substance manufacturing.

Discovery mRNA synthesis
The discovery process is all about speed and agility. You can integrate TriLink’s modified NTPs, CleanScript™ IVT method, and CleanCap® capping technology into your mRNA program. Leverage our custom mRNABuilder™ to design your mRNA or long RNA transcript, and we’ll take care of the rest.
Analytical Services for mRNA
Your mRNA program is supported by TriLink’s in-house analytics team, with extensive experience in characterizing and analyzing mRNA. Comprehensive analytical testing and quality control (QC) assays are performed to ensure the purity and integrity of the plasmid DNA template and mRNA drug substance.

Let’s discuss your mRNA program needs
Connect with our team of mRNA synthesis and manufacturing experts.


