- More Methodology from CRISPR Mania
- lncRNA Function Blocked by CRISPRi
- Mysteries of lncRNA Can Now be Deciphered by CRISPRi
This blog is about yet another example of a powerful new methodology spawned by intense scientific interest in using CRISPR-related technologies. This near mania for all things CRISPR is reflected by there being ~5,000 (!) publications already in PubMed only ~5 years after seminal papers appeared.
I chose the present blog topic because it involves use of CRISPR for genome-wide identification of functional long non-coding RNA (lncRNA) in human cells. In an earlier blog about lncRNA, which are now recognized to be regulators of gene expression encoded by what was originally defined as “junk” DNA, it was pointed out that it is inherently difficult to experimentally identify such regulation by lncRNA. Thanks to CRISPR this task is now much less daunting as you’ll learn below, following a couple of introductory sections to set the stage.
Repurposing CRISPR/Cas9 Using “Dead” Cas9
Qi et al. very cleverly—at least to me—recognized that the CRISPR/Cas9 system could be repurposed as an RNA-guided platform for sequence-specific control of gene expression by finding a catalytically inactive mutant Cas9 protein that lacked exonuclease (i.e. cutting) activity of wild-type Cas9, and instead blocked transcription by RNA polymerase (RNAP), as depicted below. These researchers coined the overall process as “CRISPR interference” (CRISPRi) and loosely referred to such a mutant Cas9 as “dead” Cas9 (dCas9).
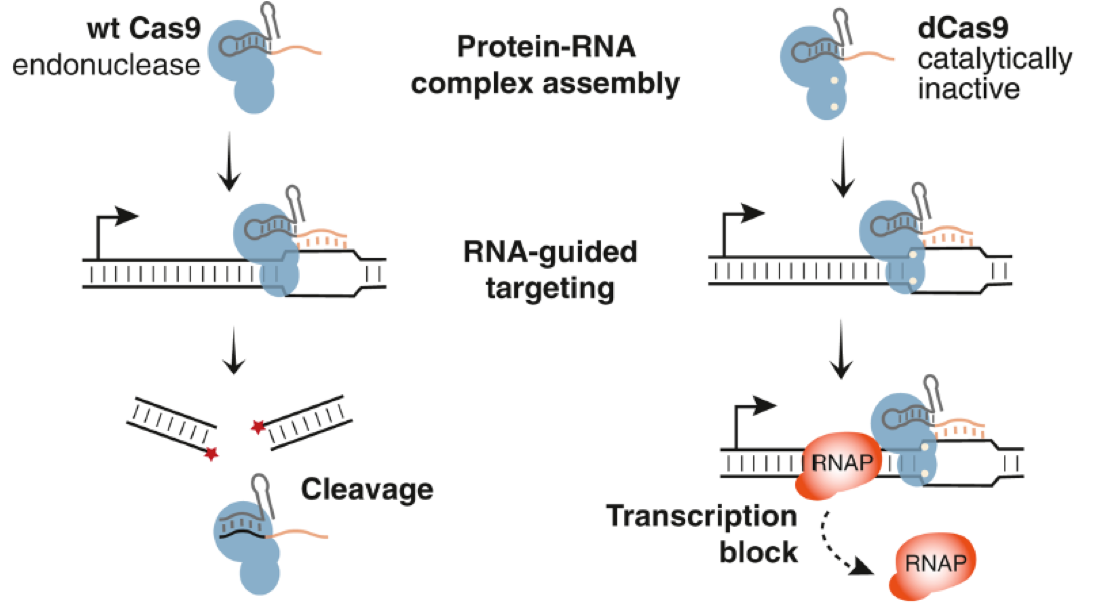 Taken from Qi et al.
Taken from Qi et al.
Interested readers are encouraged to consult this publication by Qi et al. to fully appreciate the extensive amount of work that went into translating the above concept into practice, and supporting the proposed mechanism of action. In my opinion, it’s a tour de force example of applying hypothesis-driven, state-of-the art molecular biology to devise a new method—in this case specifically blocking transcription of a DNA region using CRISPRi in conjunction with target-specific short guide RNA (sgRNA).
Adding Functionality to Down-Regulate Transcription
Just as organic chemists can design and synthesize small molecules having desired functional properties, molecular biologists can design and produce complex macromolecules having desired functional elements. The latter is nicely exemplified by Gilbert et el., who demonstrated that fusion of dCas9 to transcription factor effector domains having repressive regulatory functions enables efficient transcriptional repression in human (or yeast) cells via sgRNA that target genes of interest.
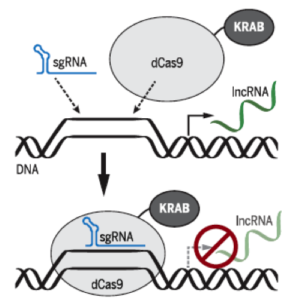 Taken from Liu et al. (2017)
Taken from Liu et al. (2017)
As depicted below, Gilbert et al. used dCas9 fused to the Krüppel associated box (KRAB) domain, which is a transcriptional repression domain, and Green Fluorescent Protein (GFP) as a reporter gene targeted by sgRNA. They employed RNA-sequencing to quantify the transcriptome of GFP-positive HEK293 cells expressing dCas9-KRAB or a negative control construct. It was shown that CRISPRi is highly specific, as GFP was the only gene that was significantly suppressed by GFP-targeting sgRNA. Averaged data from two independent biological replicates indicated that no gene other than GFP changes by >1.5-fold.
Genome-Scale CRISPRi to Identify Human lncRNA
According to Liu et al., it has not been possible to predict which lncRNA loci are functional or what function they perform. Consequently, there is a need for large-scale, systematic approaches to interrogating the functional contribution of lncRNA loci. This sizeable team of collaborators from various institutions in the San Francisco Bay area, therefore, developed a genome-scale screening platform using CRISPRi with dCas9-KRAB and a library of sgRNA.
 Taken from Liu et al. (2017)
Taken from Liu et al. (2017)
As depicted below for the overall approach, they first designed a CRISPRi Non-Coding Library, which targets 16,401 lncRNA genes each with 10 sgRNAs per transcription start site. The required 170,262 sgRNAs were not synthesized chemically, but rather produced intracellularly by first using array-based sgDNA synthesis followed by clonal (i.e. individual sgDNA sequence) incorporation into lentivirus, which in turn were transfected into seven types of cells for screening. More detail on such lentiviral libraries is given in a Footnote at the end of this blog.
As indicated pictorially above, they applied this pooled screening approach to identify lncRNA genes that modify robust cell growth for induced pluripotent stem cells (iPSC) and six well-known, transformed human cell lines (K562, U87, etc.). This led to identification of 499 lncRNA loci that modified cell growth upon CRISPRi targeting.
Interestingly—at least to me—372 (~75%) and 299 (~60%) of these 499 growth-modifying lncRNA loci were distal to a protein coding gene (PCG) or mapped enhancer, respectively. The diagram below, taken from a review by Vance & Ponting, depicts “distal” effects of lncRNA away from PCG between two chromosomes (chr). What “triggers” transcription of the lncRNA from chr A and how it “finds” its cognate PCG on chr B are open and indeed intriguing questions.

In addition to these high percentages of distal effects, Liu et al. found the following surprising results with regard to cell-type specificity of lncRNA function:
“Remarkably, 89% of the lncRNA gene hits modified growth in just one of the cell lines tested, and no hits were common to all seven cell lines. Although nearly all of the hit genes were expressed in the cell line in which they exhibited a growth phenotype, expression alone was insufficient to explain the cell type specificity of their function.”
“[Thus,>
in contrast to recent studies that found that essential protein-coding genes typically are required across a broad range of cell types, we show that lncRNA function is highly cell type-specific, a finding that has important implications for their involvement in both normal biology and disease.”
Following are some of the major unanswered questions about lncRNA posed in a review I recommend reading for more background on lncRNA:
- How does the manner in which lncRNAs are transcribed, processed, and regulated differ from that of other RNAs?
- Are lncRNAs evolutionarily conserved, both in terms of their primary sequences and secondary structures?
- Are all lncRNAs functional? Which ones have detectable biological functions in cells or in the whole organism?
- Does the pervasive transcription that generates the lncRNA transcripts play a regulatory role distinct from the steady-state accumulation of the lncRNAs?
- Can lncRNAs be exploited for clinical applications and therapeutics?
After reading this review, I thought to myself that there are many open questions about lncRNA but no comprehensive answers yet deciphered. When I then checked Google Scholar for items with both “deciphered” and “lncRNA” as terms, I found there were over 1,800 such items. Evidently, there are quite a few authors who, like me, view unknown functions of lncRNA as a cipher. I suspect that much of the now mysterious lncRNA function will eventually be deciphered thanks, in part, to the power of CRISPRi.
Your comments are welcomed.
Footnote
Readers interested in lentiviral sgRNA library construction and use for screening target cells can find general information at this website from which the following self-explanatory schematic provides a high-level overview of the workflow.







