- Polymerases Aren’t What They Used to Be!
- Scripps Team Evolves Polymerases That Read and Write With 2’-O-Methyl Ribonucleotides
- Key Reagents for Romesberg’s “Molecular Moonshots” Are Supplied by TriLink BioTechnologies
Long-time devotees of these posts will likely remember a blog several years ago about Prof. Floyd Romesberg at the Department of Chemistry, The Scripps Research Institute who achieved a seemingly impossible feat. Namely, designing a new pair of complementary bases such that DNA replicating in E. coli would be comprised of six bases, thereby creating a six-base genetic code that is expanded from Nature’s four-base code.
 Floyd E. Romesberg. Taken from utsandiego.com
Floyd E. Romesberg. Taken from utsandiego.com
More recently, Romesberg has cleverly outfoxed Nature once again, this time by evolving nucleic acid polymerases into mutant polymerases that can do what heretofore seemed impossible. He and his research team’s publication (Chen et al.) is a tour de force of experimental methodology that is not easily read, and is even harder to simply summarize in a short space like this blog. Consequently, I’ll first tell you what was accomplished, then give a short synopsis of principal new methodology, and close by commenting on the significance of this fascinating work.
Doing the Impossible
Romesberg’s lab successfully achieved what I think of as “multiple molecular moonshots,” wherein a Taq polymerase (which normally reads and writes DNA during PCR), was evolved by novel selection (SELEX) methods into mutant polymerases that are able to transcribe DNA into 2’-O-methyl (2’-OMe) RNA, and reverse transcribe 2’-OMe RNA into DNA for PCR/sequencing.
As depicted below, this was exemplified using a 60-mer DNA template and 18-mer 2’-OMe RNA primer to produce a fully-modified 48-mer 2’-OMe RNA by means of an evolved mPol and all four A, G, C and U 2’-OMe NTPs, which I’m proud to say were bought from TriLink BioTechnologies! This type of molecular evolution of a polymerase has no precedent.
DNA template 5’ ------------------------------------- 3’
RNA primer ←←← 3’ xxxxxx 5’
mPol ↓ 2’-OMe NTPs
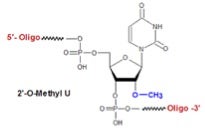
Determining the fidelity of this seemingly impossible molecular transformation was addressed by achieving a feat of comparable impossibility! As depicted below, the aforementioned 48-mer 2’-OMe RNA product was hybridized to a DNA primer for reverse transcription into a 48-mer complementary DNA (cDNA) strand, using an evolved mPol, together with all four A, G, C and T unmodified dNTPS, which were also purchased from TriLink. This unprecedented conversion of 2’-OMe RNA into cDNA was followed by conventional PCR/sequencing, the results of which demonstrated relatively high fidelity.
2’-OMe template 5’ xxxxxxxxxxxxxxxxxxxxxxxxxxx 3’
DNA primer ←←← 3’ ------ 5’
mPol ↓ dNTPs
cDNA 3’ -------------------------------------- 5’
How They Did It
In the selection cycle shown below, (1) phage-display libraries were used to expose individual polymerases (Pol) on E. coli. cells in proximity to chemically attached primer/template complexes of interest, which are mixed with natural or modified triphosphates including biotin (green; B)-labelled UTP to extend the primer. (2) Phage that display active mutant polymerases (mPols) are isolated with streptavidin (SA) beads. After washing to remove nonspecific binders, phage cleaved from the beads are used to re-infect E. coli. (3) Heat-treated lysates of E. coli that express the recovered mPols are next subjected to plate-based screening using 96-well plates coated with primer/template complex and extension buffer that contained natural or modified triphosphates and B-UTP, incorporation of which is chromogenically detected. (4) Mutants that give rise to the most activity are selected for individual gel-based analysis, from which (5) promising candidates are selected for further diversification (e.g., by gene shuffling, as depicted) and then subjected to additional rounds of evolution.
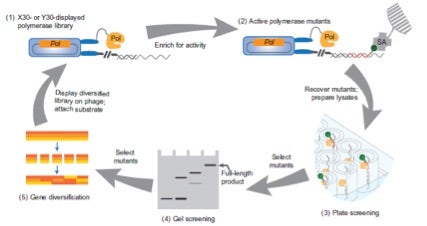 Taken from Chen et al. Nature Chemistry (2017)
Taken from Chen et al. Nature Chemistry (2017)
What is the Significance
In a previous blog, I’ve commented on increasing interest in the utility of aptamers, which are oligonucleotides that can specifically bind small molecules or motifs in proteins, and thus be used to build electronic sensors or studied as potential therapeutic agents rivaling antibodies. Therapeutic aptamers, like antisense oligonucleotides, require incorporation of chemical modifications to impart stability toward nucleases in blood or cellular targets.
Burmeister et al. have previously reported methods for mPol transcription of a DNA template into a fully modified, nuclease-resistant 23-mer 2’-OMe RNA aptamer—also using TriLink’s 2’-OMe NTPs! However, they encountered considerable experimental difficulties in generating this therapeutically promising 23-mer against vascular endothelial growth factor. These technical issues have now been surmounted by the mPol-evolution approaches in the present work by Romesberg’s team, which enabled improved access to longer 2’-OMe RNA aptamers with reasonable efficiency and fidelity.
Moreover, the present study is the first to evolve an mPol for reverse transcription of fully modified 2’-OMe RNA into DNA, which can then be amplified by PCR and/or sequenced, thereby opening the door for a variety of new analytical methods. Most importantly, the molecular mechanism by which these remarkable mPol activities was evolved, namely, the stabilization of an interaction between the “thumb and fingers domains,” may be general and thus useful for the optimization of other Pols. In that case, we can look forward to further advances in evolving other Pols to do the impossible—hopefully using modified nucleotide triphosphates from TriLink!
As usual, your comments are welcome.






