- Vaccinating the World Against COVID-19
- Karikó and Weissman Become mRNA Vaccine “Rock Stars”
- What We Know and Don’t Know About COVID-19
- First CRISPR-Cas9 Genome Editing in Humans Achieved
As 2021 draws to a close, this final blog post reflects on four topics at the top of the Zone’s RNA therapeutics trending list. As RNA therapeutics includes vaccines, the first three focus on curtailing the global COVID-19 pandemic, while the fourth, clinical CRISPR-Cas9 genome editing, addresses the therapeutic strategy of cutting DNA in vivo via Cas9 endonuclease.
Vaccinating the World Against COVID-19
The biggest vaccination campaign in history is underway. According to current vaccine tracking data, more than 8.09 billion doses have been administered across 184 countries, the latest rate being roughly 37.1 million doses a day. In the U.S., 462 million doses have been administered to date.
As of September 2021, 21 distinct anti-COVID-19 vaccines have been authorized and approved across the world:
- inactivated viruses (8)
- protein-based vaccines (4)
- recombinant adenovirus (6)
- DNA (1)
- mRNA (2)
The two mRNA vaccines, developed by Pfizer-BioNTech (brand name Comirnaty®; aka BNT162b2) and Moderna (brand name Spikevax™; aka mRNA-1273), are the most effective and widely administered (Pascolo).
According to a posted DNA sequencing study (Jeong et al.) conducted by Stanford University scientists, who used reverse-transcribed mRNA extracted from unused “leftovers” in vials of each vaccine, Comirnaty and Spikevax have similarly structured mRNA. The structural elements for mRNA in Comirnaty are shown and defined in Figure 1.

Element | Description | Position |
5’-Cap | A modified 5’-cap1 structure (m7G+m3'-5'-ppp-5'-Am) | 1-2 |
5’-UTR | Derived from human alpha-globin RNA with an optimized Kozak sequence | 3-54 |
sig | S glycoprotein signal peptide (extended leader sequence) that guides translocation of the nascent polypeptide chain into the endoplasmic reticulum | 55-102 |
S protein_mut | Codon-optimized sequence encoding full-length SARS-CoV-2 spike (S) glycoprotein, containing mutations K986P and V987P to ensure the S glycoprotein remains in an antigenically optimal pre-fusion conformation | 103-3879 |
3’-UTR | Comprises of two sequence elements derived from the amino-terminal enhancer of split (AES) mRNA and the mitochondrial encoded 12S ribosomal RNA to confer RNA stability and high total protein expression | 3880-4174 |
poly(A) | A 110-nucleotide poly(A)-tail consisting of a stretch of 30 adenosine residues, followed by a 10-nucleotide linker sequence and another 70 adenosine residues. | 4175-4284 |
FIGURE 1. Schematic diagram and structural elements of the Pfizer-BioNTech mRNA vaccine (Comirnaty). Adapted by Jerry Zon from a postings by Bert Hubert and by Jeong et al., who provide the full sequence for both Comirnaty and the Moderna vaccine (Spikevax), as derived from DNA sequencing of each reverse-transcribed mRNA vaccine.
Manufacturing Billions of Doses of COVID-19 mRNA Vaccines
The speed with which development and initial manufacturing of these vaccines occurred was the result of unprecedented efforts and coordination between these companies, their supply chain partners, health care systems, and governments worldwide. In 2021, the daunting challenge became the enormously large scale-up required to manufacture billions of doses of Comirnaty and Spikevax. To put this scale-up into perspective, consider the following series of calculations:
in February 2021, BioNTech announced it would increase production of Comirnaty by over 50%, manufacturing 2 billion (109) doses in 2021, which was then raised again in March to 2.5 billion doses. The approved adult dose is 30 micrograms (10-6 g; µg) of active pharmaceutical ingredient (API). Multiplying 30 x 10-6 g per dose by 2.5 x 109 doses equals 75 x 103 g (75 kilograms; kg). Thus, BioNTech intended to produce 165 pounds of the Comirnaty mRNA API in 2021.
To a manufacturing chemist familiar with synthesizing small molecules, 75 kg of API is not uncommon, and in some cases, it is actually small scale. To a biochemist or molecular biologist familiar with enzyme-mediated in vitro transcription (IVT) synthesis of macromolecular mRNA, however, 75 kg of mRNA API is truly a gigantic amount of material. For example, a typical 1-mL IVT reaction yields 5 x 10-3 (mg) of crude mRNA (Henderson et al.). This product would then be purified, further reducing the final yield. Thus, the above-mentioned 75 kg of mRNA API represents the equivalent of 15 million 1-mL scale IVT reactions, without the expected losses during purification, formulation, vial filling, etc. Therefore, the scale up that successfully achieved production of 2.5 billion doses of Comirnaty during 2021 was an unprecedented achievement.
In February 2021, Moderna announced it was increasing its 2021 base plan to 700 million 100-µg doses of Spikevax, with the goal of supplying up to 1 billion doses in 2021. Using the same calculations as outlined above, these 1 billion doses are equal to 100 kg or 220 pounds of the Spikevax mRNA API in 2021, representing the equivalent of 20 million 1-mL scale IVT reactions.
Combined, the 385 pounds of mRNA API for the Comirnaty and Spikevax vaccines in 2021 represent a truly laudatory achievement by the companies, suppliers, and contactors involved.
The Zone is proud to note that this includes TriLink! With state-of-the-art facilities as a Contract Development and Manufacturing Organization (CDMO), TriLink has ongoing contracts with leading pharmaceutical companies to provide TriLink’s proprietary CleanCap® technology (Figure 2).
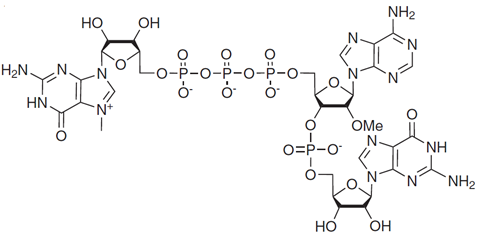
FIGURE 2. Structure of CleanCap® AG trimer. Taken from Henderson et al. Current Protocols, 1, e39. Open Access and free to use (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Importantly, the number of mRNA vaccines targeted for manufacture next year are even greater than those mentioned above: BioNTech and Moderna each intend to produce 3 billion doses (90 kg or 200 pounds of Comirnaty and 300 kg or 660 pounds of Spikevax) of their vaccine in 2022. This goal is driven by the desire to expand the global percentage of vaccinated persons (currently 56%), support booster shot administration, and provide vaccines for newly eligible age groups.
Karikó and Weissman Become mRNA Vaccine “Rock Stars”
For many, mRNA vaccines seemed to have materialized very quickly in response to the COVID-19 pandemic. However, the history of mRNA vaccine development is long and ‘tangled,’ as outlined in a September 2021 Nature news feature. As is the case with many fields, hundreds of scientists worked on mRNA vaccines for decades before the coronavirus pandemic led to a breakthrough. Now, there are disparate opinions as to “who did what, when” and who deserves recognition.
The current debate among vaccinology experts includes the critically important contributions made by Katalin Karikó and Drew Weissman, pictured below. Their collaborative studies at the University of Pennsylvania in the early 2000s pioneered the use of nucleoside-modified mRNA, embodied in both Comirnaty and Spikevax formulations. Before discussing the particular nucleoside modification, the backstory for the serendipitous Karikó and Weissman collaboration is worth summarizing, as Karikó persevered in the pursuit of mRNA therapeutics, despite many barriers.

Katalin Karikó, PhD (with permission) Drew Weissman, MD PhD (commons.wikimedia.org)
As detailed in a Washington Post story, Karikó obtained her PhD degree by working with RNA at the University of Szeged after growing up in a two-room adobe house with a thatched roof in the small village of Kisujszallas in Hungary. Her father was a butcher, her mother a bookkeeper. There was no running water, no television, and no refrigerator in her childhood home. In 1985, the lab where Karikó worked lost its funding. She looked for an opportunity in the U.S. and found a postdoc position at Temple University in Philadelphia.
In the late 1990s, Karikó happened to meet a faculty member named Drew Weissman while using a photocopier at Penn. Weissman had been a postdoc with the now famous Anthony Fauci at NIH, and he wanted to create an HIV vaccine. She told him about mRNA, touting its vast potential, and offered to make mRNA for one of Weissman’s experiments. When Weissman tested it in specialized immune cells, he found that the mRNA triggered an unwanted inflammatory response—a blow for Karikó. Solving that problem represented the start of what would become a world-changing scientific collaboration.
In 2005, Karikó and Weissman showed that unmodified RNA, comprised of A, G, C, and U, signals the innate immune system through human toll-like receptors (Figure 3), but incorporation of modified nucleosides found in transfer RNA reduces this immune-stimulation (Karikó et al. 2005). Antigen- presenting dendritic cells exposed to nucleoside-modified RNA expressed significantly less cytokines and activation markers than those treated with unmodified RNA. The researchers concluded that the innate immune system may react to RNA lacking nucleoside modification as a means of selectively responding to bacteria or necrotic tissue, adding that their findings could “give future directions into the design of therapeutic RNA.”
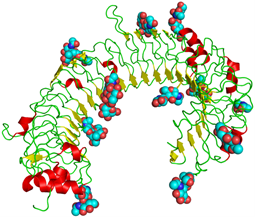
FIGURE 3. Structure of toll-like receptor 3 (TLR3). Attached sugars (spheres) and internal structures (wires, arrows, and helixes). Taken from commons.wikimedia.org and free to use.
Three years later, Karikó et al. 2008 reported that, among several types of naturally occurring nucleoside-modified IVT Renilla luciferase–encoding mRNAs, those containing pseudouridine (ψ) had a 10-times higher translational capacity than unmodified mRNAs when transfected with lipofectamine into various cell types, including mouse embryonic fibroblasts, splenocytes, and human DCs.
Like ψ, N1-methylpseudouridine (m1ψ) is also a natural tRNA component, differing from ψ only by replacement of the N1 hydrogen with a methyl group. Consequently, Karikó and Weissman extended their investigations of ψ-modified mRNA to m1ψ-modified mRNA, which led to a 2015 publication with collaborators (Pardi et al.). The promising results with m1ψ-modified mRNA were prepared by IVT using m1ψ-5’-triphosphate (Figure 4) obtained from TriLink.
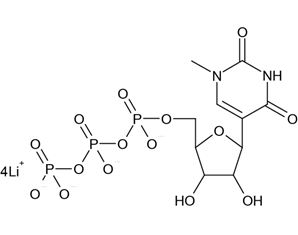
FIGURE 4. Structure of N1-methylpseudouridine-5’-triphosphate, lithium salt. Taken from TriLink BioTechnologies.
In closing this section, the Zone extends congratulations to Karikó and Weissman for their above-mentioned pioneering studies of nucleoside-modified mRNA as vaccines and for other therapies:
- August 16, 2021: The 2021 Louisa Gross Horwitz Prize “for pioneering research on messenger RNA vaccines for COVID-19.” Of the 106 Horwitz Prize winners to date, 51 have gone on to receive Nobel Prizes.
- September 9, 2021: The 2022 Breakthrough Prize in Life Sciences “the innovative vaccines developed by Pfizer/BioNTech and Moderna that have proven effective against the virus rely on decades of work by Katalin Karikó and Drew Weissman.”
- September 24, 2021: The 2021 Lasker-DeBakey Clinical Medical Research Award “for the discovery of a new therapeutic technology based on the modification of messenger RNA—enabling rapid development of highly effective Covid-19 vaccines.” Dozens of Lasker awardees have gone on to be awarded Nobel prizes.
What We Know and Don’t Know About COVID-19 in 2021
Currently, more than 10,000 articles published in PubMed in 2021 include “COVID-19 or SARS-CoV-2” and “vaccine” in the title/abstract. This highly active area of research is rapidly expanding our knowledge of the SARS-CoV-2 virus and its effects on the planet. Broadly speaking, the new “knowns” about COVID-19 for 2021 can be grouped into three general categories—epidemiology, immunology, and virology:
Epidemiology: the statistics vary from country to country. In general, based on data provided by the CDC, deaths in 2020/2021 involving COVID-19 increase with age: 18-29 years, 3888; 30-39 years, 11313; 40-49 years, 28190; 50-64 years, 125812; 65-74 years, 160596; ≥85 years, 195007. The number of COVID-19 cases per 100,000 persons however, is shifting from older to younger groups.
Immunology: immunological studies of patients hospitalized with COVID-19 show that early presence of broadly functional antibodies directed at the SARS-CoV-2 spike (S) protein (Figure 5) correlate with survival, and S-protein-targeted neutralizing antibodies are present in the majority of individuals following infection. Researchers now know that B cells make antibodies against SARS-CoV-2, while also understanding the importance of T cells in thwarting infections. Helper T cells spur B cells and other immune defenders into action, whereas killer T cells target and destroy infected cells. Severity of disease can depend on the strength of these T cell responses.
Virology: molecular-level virology studies (Yang et al.) have shown that SARS-CoV-2 entry into host cells is mediated by its S protein, and the angiotensin-converting enzyme 2 (ACE2) has been identified as a cellular receptor (Figure 5). Structural analysis by cryo-electron microscopy (CryoEM) or X-ray crystallography has revealed the structure of 6 viral proteins (S protein, NSP1, ORF3a, ORF9b, E and N proteins) in complex with human proteins. More than 1000 proteins have interaction partners with these viral proteins. By interacting with host cell proteins, SARS-CoV-2 disrupts normal cellular function and employs cellular equipment to replicate itself (Jahanafrooz et al.)
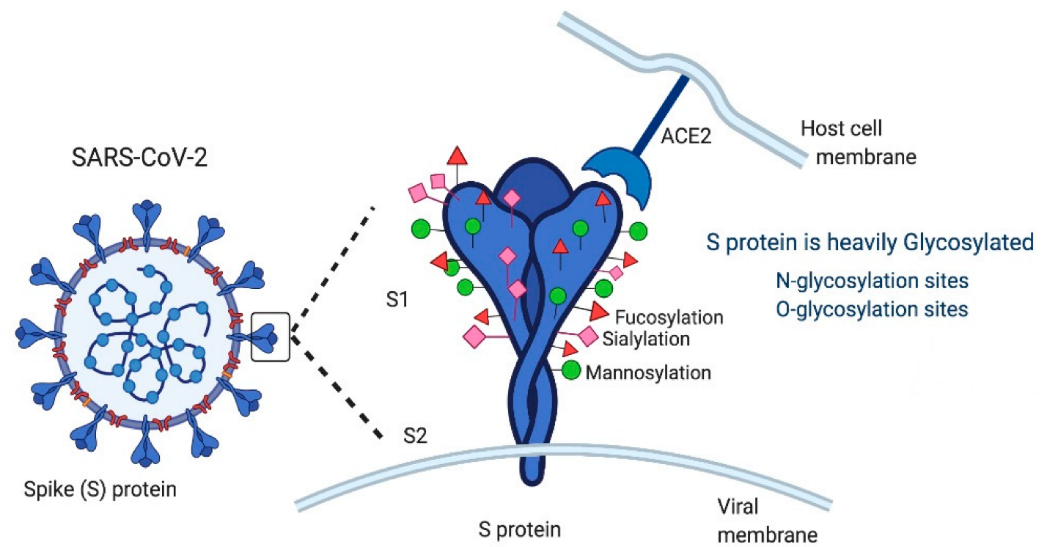
FIGURE 5. Schematic representation of SARS-CoV-2 virus showing the spike (S) protein. SARS-CoV-2 is a lipid-enveloped positive-sense RNA virus that uses a heavily glycosylated spike (S) protein to facilitate its attachment, membrane fusion, and entry into host cells. Taken from Gadanec et al. Int. J. Mol. Sci. 2021, 22, 992. Copyright: © 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license https://creativecommons.org/licenses/by/4.0/.
Among the “unknowns,” the following are a sampling of open questions based on a Johns Hopkins School of Public Health expert-interview published on June 23, 2021:
How much immunity is enough immunity to COVID to avoid symptoms, and can we measure this from previous infections or vaccinations?
The level of COVID-19 antibodies someone has can be measured after infection and after vaccination. Scientists also know that T cell responses are very important to fight COVID-19. However, we do not yet understand the levels of antibodies or T-cell responses required for “sufficient” immunity.
What causes “long-COVID” that some people experience weeks or months after their initial infection clears?
The NIH hopes to learn the unknown causes of “long COVID” through a recently initiated clinical study of 40,000 adults. The goal is to collect ‘lots of data about symptoms, physical findings, and laboratory measures,’ according to NIH Director Francis Collins in a Science report; more data will help us understand this aspect of the disease better.
What do we know about rare instances of myocarditis or heart inflammation in some teens after COVID-19 vaccination?
The Zone found numerous publications on this apparent and rare adverse reaction, but so far there is no direct link. The CDC is reporting that the cases have been mild, resolving with medicine and rest.
The Spread of the Delta Variant
In 2021, the SARS-CoV-2 Delta variant emerged. In brief, Delta is more infectious and transmissible than earlier forms of the virus. Now, at the end of 2021, there are more than 360 publications in PubMed indexed to “Delta variant” in the title/abstract.
One study (Liu et al.), published in September, studied the spike protein of the Delta variant. Briefly, Liu et al. compared Delta to the original Alpha variant and showed that spike mutations are responsible for the enhanced viral fitness of the Delta variant. In October, the structures of the S proteins of the Delta, Kappa, and Gamma variants were determined by CryoEM (Zhang et al.). Comparison of the structure, function and antigenicity of the Delta S with those of Gamma and Kappa, and previously characterized Alpha and Beta, provided molecular insights into mechanisms of the heightened transmissibility and enhanced immune evasion of the most contagious form of SARS-CoV-2 since its initial outbreak (Zhang et al.).
While COVID-19 will undoubtedly be an ongoing health problem, its severity will hopefully decrease with time. As the pandemic wanes, additional mRNA vaccines and RNA-enabled genome editing research can expand again, leading the way for new RNA-based therapeutic discoveries.
First CRISPR-Cas9 Genome Editing in Humans Achieved
A team of researchers, including those from Intellia Therapeutics and Regeneron Pharmaceuticals, published the first-ever clinical data supporting the safety and efficacy of CRISPR-Cas9 genome editing in humans (Gillmore et al.). These findings have been heralded as opening a ‘new era of medicine.’ NTLA-2001, the lead drug candidate, generated a dose-dependent and sustained reduction of its target, a protein linked to transthyretin amyloidosis (ATTR). The study was done in six patients living with hereditary transthyretin amyloidosis with polyneuropathy (ATTRv-PN) and clinical response was observed after a single dose.
ATTR amyloidosis is a progressive fatal disease characterized by the accumulation of misfolded transthyretin (TTR) protein amyloid fibrils in tissues (Figure 6). ATTR amyloidosis can be de novo acquired or hereditary. With a global incidence of 50,000 persons, hereditary ATTR can be caused by over 100 different pathogenic mutations in the TTR gene. After symptom onset, the average lifespan is 2-6 years for patients affected by ATTR-cardiomyopathy, and 4-17 years for those affected by ATTR-polyneuropathy.
NTLA-2001 is comprised of a proprietary LNP delivery system with liver tropism, carrying a single guide RNA (sgRNA) that targets human TTR gene and a human-codon–optimized mRNA sequence of Streptococcus pyogenes Cas9 protein. Plasma apolipoprotein E binds to the LNP surface in circulation, and the LNP is then actively endocytosed by hepatocytes through the low-density lipoprotein (LDL) receptor (Figure 7). As the liver almost exclusively synthesizes TTR protein, this liver-targeting delivery system should maximize efficacy while minimizing toxic effects.

FIGURE 7. Low-density lipoprotein (LDL) particles binding to LDL receptors on the cell membrane. The binding of LDL particles (spheres) to the LDL receptors mediates the endocytosis of the particles through clathrin coated vesicles (seen in foreground and lower Right); 3D rendering.
This Phase 1 study is ongoing. Dose escalation continues, and the goal is greater reductions in serum TTR protein than those achieved with available therapies, with anticipated beneficial effects on disease progression, quality of life, and mortality. The investigative team concluded that “data from the initial groups of patients in this study provide clinical proof-of-concept for in vivo CRISPR-Cas9–mediated gene editing as a therapeutic strategy.”
Concluding Comments
The global pandemic has inflicted a terrible toll on life, but there is some solace in recognizing that the incredibly rapid development of Comirnaty and Spikevax mRNA vaccines has saved countless lives. From a nucleic acid chemistry perspective, the critical role of the m1ψ nucleoside modification in these highly effective IVT mRNAs—and future vaccines under development—has been rightfully reflected by the recognition of the pioneering contributions by Katalin Karikó and Drew Weissman.
Over the past several decades, advances in nucleic acid chemistry have transformed molecular medicine through innovations in antisense oligonucleotides, short-interfering RNAs, and more recently, nucleoside-modified mRNAs and CRISPR gene editing. The COVID-19 modified mRNA vaccines and clinical success of NTLA-2001 are opening new and exciting eras in vaccinology and medicine.
What do you think?
Your comments are welcomed, as usual.
Please feel free to share this blog with your colleagues or on social media.