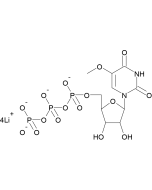
N1-Methylpseudouridine-5'-Triphosphate - (N-1081)
N1-Methylpseudouridine-5'-Triphosphate, sodium salt, is a modified NTP for incorporation into messenger RNAs (mRNA) using T7 RNA Polymerase. Incorporation of N1-Methylpseudouridine can reduce the immunogenicity of the resulting mRNA.
To learn more about TriLink's N1-Methylpseudouridine, visit https://www.trilinkbiotech.com/modified-nucleotides
| Catalog No | N-1081 |
|---|---|
| Purity | ≥99% by AX-HPLC |
| Extinction Coefficient | 8,877 Lmol-1cm-1 at 271 nm |
| Molecular Formula | C10H17N2O15P3 (free acid) |
| Molecular Weight | 498.10 g/mole (free acid) |
| Salt Form | Na+ |
| Concentration | 100 mM |
| Buffer | H2O |
| Recommended Storage | -20°C or below |
| Other Name(s) | N1-Methylpseudouridine-5'-Triphosphate, N1meΨTP, m1ΨTP, 1-Methyl-PseudoUridine Phosphoramidite, N1-Methyl-Pseudouridine-5'-Triphosphate |
| Application | In vitro Transcription |
| Backbone | 5'-Triphosphate |
| Base Analog(s) | Pseudouridine |
| Sugar Type(s) | RNA |
| Nucleotide Category | Base Modified RNA |
N-1081 Safety Data Sheet
Ask an Expert
CoA search tool
Products are for research use only, not for use in diagnostic or therapeutic procedures or for use in humans. Products are not for resale without express written permission from TriLink No license under any patent or other intellectual property right of TriLink or its licensors is granted or implied by the purchase unless otherwise provided in writing.
TriLink does not warrant that the use or sale of the products delivered hereunder will not infringe the claims of any United States or other patents or patents pending covering the use of the product alone or in combination with other products or in the operation of any process. All and any use of TriLink product is the purchaser's sole responsibility.
- Pardi, Norbert; Tuyishime, Steven; Muramatsu, Hiromi; Kariko, Katalin; Mui, Barbara L.; Tam, Ying K.; Madden, Thomas D.; Hope, Michael J.; Weissman, Drew . Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes.
- Pardi, Norbert; Parkhouse, Kaela; Kirkpatrick, Ericka; McMahon, Meagan; Zost, Seth J.; Mui, Barbara L.; Tam, Ying K.; Karikó, Katalin; Barbosa, Christopher J.; Madden, Thomas D.; Hope, Michael J.; Krammer, Florian; Hensley, Scott E.; Weissman, Drew . Nucleoside-modified mRNA immunization elicits influenza virus hemagglutinin stalk-specific antibodies.
- Tiwari, Pooja Munnilal; Vanover, Daryll; Lindsay, Kevin E.; Bawage, Swapnil Subhash; Kirschman, Jonathan L.; Bhosle, Sushma; Lifland, Aaron W.; Zurla, Chiara; Santangelo, Philip J. . Engineered mRNA-expressed antibodies prevent respiratory syncytial virus infection.
- Laczk . A single immunization with nucleoside-modified mRNA vaccines elicits strong cellular and humoral immune responses against SARS-CoV-2 in mice
- Lockhart, JH;VanWye, J;Banerjee, R;Wickline, SA;Pan, H;Totary-Jain, H; . Self-assembled miRNA-switch nanoparticles target denuded regions and prevent restenosis
- Leppek, K;Byeon, GW;Kladwang, W;Wayment-Steele, HK;Kerr, CH;Xu, AF;Kim, DS;Topkar, VV;Choe, C;Rothschild, D;Tiu, GC;Wellington-Oguri, R;Fujii, K;Sharma, E;Watkins, AM;Nicol, JJ;Romano, J;Tunguz, B;Participants, E;Barna, M;Das, R; . Combinatorial optimization of mRNA structure, stability, and translation for RNA-based therapeutics
- Chen, PJ;Hussmann, JA;Yan, J;Knipping, F;Ravisankar, P;Chen, PF;Chen, C;Nelson, JW;Newby, GA;Sahin, M;Osborn, MJ;Weissman, JS;Adamson, B;Liu, DR; . Enhanced prime editing systems by manipulating cellular determinants of editing outcomes
- Everton, E;Rizvi, F;Smith, AR;Beattie, M;Tam, Y;Pardi, N;Weissman, D;Gouon-Evans, V; . Transient yet Robust Expression of Proteins in the Mouse Liver via Intravenous Injection of Lipid Nanoparticle-encapsulated Nucleoside-modified mRNA
- Appelberg, S;John, L;Pardi, N;V . Nucleoside-modified mRNA vaccines protect IFNAR-/- mice against Crimean Congo hemorrhagic fever virus infection
- Matias, J;Kurokawa, C;Sajid, A;Narasimhan, S;Arora, G;Diktas, H;Lynn, GE;DePonte, K;Pardi, N;Valenzuela, JG;Weissman, D;Fikrig, E; . Tick immunity using mRNA, DNA and protein-based Salp14 delivery strategies
- Svitkin, YV;Gingras, AC;Sonenberg, N; . Membrane-dependent relief of translation elongation
- Parhiz, H;Brenner, JS;Patel, P;Papp, TE;Shahnawaz, H;Li, Q;Shi, R;Zamora, M;Yadegari, A;Marcos-Contreras, OA;Natesan, A;Pardi, N;Shuvaev, VV;Kiseleva, R;Myerson, J;Uhler, T;Riley, RS;Han, X;Mitchell, MJ;Lam, K;Heyes, J;Weissman, D;Muzykantov, V; . Added to pre-existing inflammation, mRNA-lipid nanoparticles induce inflammation exacerbation (IE)
- Whitley, J;Zwolinski, C;Denis, C;Maughan, M;Hayles, L;Clarke, D;Snare, M;Liao, H;Chiou, S;Marmura, T;Zoeller, H;Hudson, B;Peart, J;Johnson, M;Karlsson, A;Wang, Y;Nagle, C;Harris, C;Tonkin, D;Fraser, S;Capiz, L;Zeno, CL;Meli, Y;Martik, D;Ozaki, DA;Caparoni, A;Dickens, JE;Weissman, D;Saunders, KO;Haynes, BF;Sempowski, GD;Denny, TN;Johnson, MR; . Development of mRNA manufacturing for vaccines and therapeutics: mRNA platform requirements and development of a scalable production process to support early phase clinical trials
- Aditham, A;Shi, H;Guo, J;Zeng, H;Zhou, Y;Wade, SD;Huang, J;Liu, J;Wang, X; . Chemically Modified mocRNAs for Highly Efficient Protein Expression in Mammalian Cells
- Ge, N;Sun, J;Liu, Z;Shu, J;Yan, H;Kou, Z;Wei, Y;Jin, X; . An mRNA vaccine encoding Chikungunya virus E2-E1 protein elicits robust neutralizing antibody responses and CTL immune responses
- Liu, W;Alameh, MG;Yang, JF;Xu, JR;Lin, PJC;Tam, YK; . Lipid nanoparticles delivering constitutively active STING mRNA as a novel anti-cancer therapeutic approach
- Kaur, K;Hadas, Y;Kurian, AA;?ak, MM;Yoo, J;Mahmood, A;Girard, H;Komargodski, R;Io, T;Santini, MP;Sultana, N;Kabir Sharkar, MT;Magadum, A;Fargnoli, A;Yoon, S;Chepurko, E;Chepurko, V;Eliyahu, E;Pinto, D;Lebeche, D;Kovacic, JC;Hajjar, RJ;Rafii, S;Zangi, L; . Direct Reprogramming Induces Vascular Regeneration Post Muscle Ischemic Injury
- Nakanishi, H;Saito, H; . Purification of Specific Cell Populations Differentiated from Stem Cells Using MicroRNA-Responsive Synthetic Messenger RNAs