This blog discusses a recent report by researchers (Cui et al.) in Toronto, Canada, who describe an AI-driven autonomous system for accelerating the discovery of new ionizable lipids for mRNA delivery. This autonomous “self-driving laboratory” is named LUMI-lab, which stands for Large-scale Unsupervised Modeling followed by Iterative experiments. LUMI-lab incorporates large-scale molecular pretraining, active learning, and robotic automation to enable efficient exploration of new chemical space.
As outlined in the following sections, the development of LUMI-lab used TriLink’s CleanCap Firefly Luciferase (FLuc) mRNA, a reporter enzyme, and TriLink’s newly introduced CleanCap M6 CRISPR-Associated Protein 9 (Cas9) mRNA, modified with N1-methylpseudouridine. CleanCap M6 has a methyl group on position 6 in an adenosine in the 5’ cap structure and substantially increases protein expression by inhibiting decapping and subsequent degradation of mRNA (Mandell & Ujita et al.).
Closed-loop optimization and autonomous lab for ionizable lipid engineering
Lipid nanoparticles (LNPs) for mRNA delivery typically consist of four key components: ionizable lipids, cholesterol, helper lipids, and PEGylated lipids. Among these, ionizable lipids play an essential role in efficient RNA encapsulation and facilitating endosomal escape, a crucial step in cytoplasmic mRNA release (Han et al.).
Given this essential role in LNPs, Cui et al. designed LUMI-lab to discover and optimize ionizable lipids for enhanced mRNA delivery. At the core of the system is the LUMI-model, a pretrained foundation model, which is derived from 28 million 3D molecular structures and related physicochemical properties, and serves as the computational “brain” of LUMI-lab.
In brief, each experimental iteration begins with LUMI-model proposing a set of chemically diverse ionizable lipid structures. These lipid candidates are then synthesized (18 h) in a high-throughput manner by an automated liquid handler (Figure 1). The resulting ionizable lipids are transferred to a second liquid handler and formulated into LNPs with helper lipids, cholesterol, PEG-lipids, and CleanCap FLuc mRNA, using a classic LNP formulation ratio (Kauffman et al.).
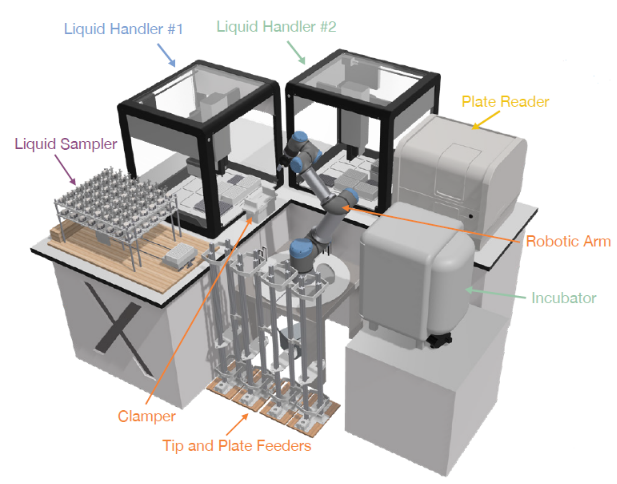
FIGURE 1.LUMI-lab hardware modules and the automatic experiments controlled by the orchestration module. Image taken from Cui et al. and used under CC BY-NC-ND 4.0 license.
Next, 96-well plates containing cultured cells are then treated with mRNA-LNP samples using a liquid handler, followed by cell incubation (18 h), after which a robotic arm transfers the plates to a liquid handler for addition of a luciferase substrate to measure bioluminescence signal intensity by a plate reader, thereby quantifying FLuc mRNA transfection efficiency.
This enables determination of mRNA transfection potency (mTP) for LNPs with varying ionizable lipids. The data from each experimental cycle (39 h) are processed automatically by the LUMI-lab’s software modules, which handle error correction, quality control, data normalization, and iterative updates to LUMI-model using active learning algorithms. This closed-loop approach systemically integrates experimental insights for optimizing LUMI-model’s performance, thereby resulting in improvements in mTP by successive iterations, as discussed in the next section.
LUMI-lab finds new effective LNPs for bronchial epithelial cell transfection in ten iterations
Because pulmonary administration by inhalation is attractive compared to injections, Cui et al. utilized LUMI-lab to conduct consecutive design-make-test-analyze iterations to optimize LNPs for mRNA delivery to human bronchial epithelial (HBE) cells.
Each iteration comprised two parallel experiments: one 96-well plate for exploration of new structures and one 96-well plate for exploitation of found outperformers. This combined exploration-exploitation approach involved synthesis of 184 lipid candidates followed by LNP formulation and evaluation. Each plate included two wells treated by industry-standard LNPs containing the benchmark ionizable lipid DLin-MC3-DMA (MC3) and two non-treated wells as negative controls.
Throughout 10 iterations, LUMI-lab evaluated a total of 1,781 distinct lipid candidates, achieving substantial improvement in mTP-measured mRNA transfection efficiency. Early iterations prioritized refining the predictive accuracy of LUMI-model, as the experimental data revealed more structure activity relations (SARs) between molecular features and transfection performance.
By the fourth iteration, the system identified several promising lipids that outperformed positive controls. Notably, performance gains accelerated in later iterations, driven by the combined effects of refined exploitation and data-informed exploration. The tenth and final iteration demonstrated LUMI-model’s ability to consistently propose high-efficacy lipids: ˃50% of candidates exhibited ˃10-fold higher transfection efficiencies.
Analysis of the ten iterations revealed key trends indicative of LUMI-model’s learning process. The exploitation plates consistently yielded high-performing candidates, while exploration plates expanded the diversity of chemical features captured by the model. By later iterations, LUMI-lab converged on specific h
eadgroups and linker structures associated with high transfection potency. Cui et al. suggest that these structures influence lipid packing and facilitate endosomal escape, critical factors for effective mRNA transfection.
They added that these findings underscore the importance of integrating exploration into active learning workflows, particularly in sparse and underexplored chemical spaces, where expanding structural diversity is essential for identifying novel, high-performance molecules. Such discovery is exemplified in the next section.
A new family of effective ionizable lipids with brominated tails discovered by LUMI-lab
Throughout the active learning and fine-tuning process, LUMI-model progressively learned the relationship between molecular features of ionizable lipids and their mRNA transfection performance. This relationship was visualized through UMAP projections (a dimensionality reduction technique) of lipids generated by the optimized LUMI-model after the tenth iteration. In the UMAP of the entire 221,000 synthesizable lipid chemical space, lipids with the highest and lowest predicted mTPs form distinct local clusters, reflecting well-captured SARs.
Notably, one of the most prominent clusters corresponds to lipids with brominated tails. Further analysis revealed that this brominated lipid cluster is enriched in lipids with globally high predicted mTP and low prediction-variance, suggesting a strong correlation between bromine modification and mRNA transfection performance.
Beyond identifying brominated lipids as a distinct structural-space feature, LUMI-model actively prioritized these lipids in experimental iterations. Despite bromine-tail lipids accounting for only approximately 8% of the initial 221,000 synthesizable lipids, more than half of all top performing lipids (i.e., top 10% mTPs) across experiments were brominated, confirming their impactful contribution to enhanced mRNA transfection efficiency.
However, while bromination contributes substantially to mTP, headgroups and linkers also have an influence, as discussed in the next section.
Enhanced in vivo mRNA delivery and gene editing potential of brominated ionizable lipids
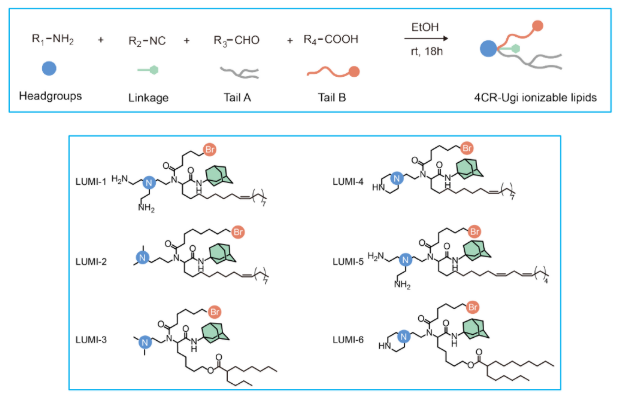
While LUMI-lab successfully identified high-mTP lipids and uncovered brominated tails as a key feature enhancing LNP performance in in vitro studies, Cui et al. sought to validate the functional consistency of these findings in more complex physiological environments. To this end, they conducted an in vivo study of pulmonary mRNA delivery, evaluating the top-performing lipids from LUMI-lab’s final iteration. Specifically, as shown in Figure 2, the six highest-mTP ionizable lipids (LUMI-1 thru LUMI-6) were selected, all of which interestingly contained brominated tails.
FIGURE 2. Top: Four components of a generalized 4CR-Ugi synthesis of ionizable lipids. Bottom: Six highest-mTP lipids with brominated (Br) tails. Image taken from Cui et al. and used under CC BY-NC-ND 4.0 license.
Characterization of nanoparticle size and polydispersity index confirmed that all six of the resultant LNPs formed stable complexes with known helper lipid systems and successfully encapsulated CleanCap FLuc mRNA. LNPs were intratracheally administered into mouse lungs via intratracheal and the bioluminescent images of the lungs were captured 6-h post-injection to assess in vivo mRNA transfection efficiency.
Notably, five candidates exhibited mRNA delivery efficiency comparable to or exceeding SM-102, an industry-standard lipid used in Moderna’s COVID-19 mRNA vaccine, while the least effective candidate still matched the performance of MC3, another FDA-approved ionizable lipid, which was mentioned above.
To assess whether the enhanced mRNA transfection efficiency of the best-performing LUMI-6 was attributable to bromine incorporation, a debrominated version (LUMI-6D) lacking bromine was synthesized. LNPs were formulated under identical conditions to ensure experimental consistency. Bioluminescence assays in HBE cells revealed that LUMI-6 exhibited 1.8-fold higher mRNA transfection efficiency than LUMI-6D, directly confirming that bromine incorporation plays a critical role in enhancing mRNA delivery.
Building on these findings, Cui et al. further investigated the potential of LUMI-6 for CRISPR-Cas9 gene editing in the lung. LUMI-6 LNPs encapsulating CleanCap M6 Cas9 mRNA and guide RNA (gRNA)were formulated and intratracheally administered into genetically engineered Ai9 reporter mice, which are frequently used for gene editing studies. In these mice, gRNA directs the Cas9 enzyme to trigger the disruption of stop signals and thereby turn on expression of red-orange fluorescent tdTomato protein.
After 9 days, flow cytometry analysis of tdTomato-positive cells showed that LUMI-6 LNP achieved a gene editing efficiency of approximately 20% in lung epithelial cells, a substantial improvement over SM-102 LNP. To assess the distribution of CRISPR-Cas9 editing mediated by LUMI-6 LNPs across cell subtypes, Cui et al. performed immunofluorescence staining of ciliated cells and club cells, two important airway epithelial populations implicated in lung diseases such as cystic fibrosis. The results confirmed efficient gene editing in both cell types, thus reinforcing LUMI-6’s potential for gene editing in the airway epithelium
Concluding Comments
A notable finding of this study is LUMI-lab’s autonomous identification of brominated lipid tails as a novel structural feature that enhances mRNA delivery, an insight previously unrecognized in expert-driven LNP design. This discovery underscores the power of AI-guided autonomous platforms to reveal new fundamental design principles beyond human intuition.
From a therapeutics perspective, LUMI-6 exhibited a preference for transfecting lung epithelial cells, which is relevant for gene therapy targeting lung congenital diseases such as cystic fibrosis, alpha-1 antitrypsin deficiency, and surfactant protein disorders, where precise gene correction in airway epithelial cells is critical.
Your comments are welcomed, as usual.
Please feel free to share this blog with your colleagues or on social media.