- Biosynthetic modified mRNA for gene-based therapy without the gene!
- AstraZeneca bets up to $420M on Moderna’s “messenger RNA therapeutics”
- “Me-too” Pharma frenzy to follow?
In a perspective on gene therapy published in Science this year, Inder M. Verma starts by observing that the concept of gene therapy is disarmingly simple. Introduce a healthy gene in a patient and its protein product should alleviate the defect caused by a faulty gene or slow the progression of the disease. He then asks the rhetorical question: ‘why then, over the past three decades, have there been so few clinical successes in treating patients with this approach?’ The answer in part has to do with challenges for cell or tissue-specific delivery, which admittedly is an issue for virtually any type of therapeutic agent. There is also concern for adverse events generally ascribed to unintended vector integration leading to neoplasias. Nevertheless, according to Verma, the present clinical trials pipeline is jammed with more than 1700 (!) clinical trials worldwide, drawing on a wide array of gene therapy approaches for both acquired and inherited diseases.
In view of this scientifically laudable but undeniable—if not frustratingly—slow progress, it’s not surprising that various groups of investigators—and investors—have recently opted to pursue a strategy that eliminates a DNA-encoded gene entirely! Instead, biosynthetic mRNA is delivered in order to directly produce the desired therapeutic protein product—this is now being referred to as “mRNA therapeutics”.
Having said this, let’s consider some pivotal scientific publications, patents, and the emerging commercial landscape for what looks to be a very hot area for research and corporate competition.
Modified mRNA Therapeutic Vaccines
An excellent review published in 2010 by Bringmann et al. entitled RNA Vaccines in Cancer Treatment covers various approaches to using mRNA encoding for tumor-associated antigens to induce specific cytotoxic T lymphocyte and antibody responses. RNA-transfected dendritic cell vaccines have been extensively investigated and are currently in numerous clinical trials (the details for which can be found at the NIH ClinicalTrials.gov website by simply searching RNA vaccines).
Interestingly, clinical feasibility and safety assessment for direct intradermal injection of “naked” unmodified mRNA was reported back in 2008 by Weide et al., who removed metastatic tissue from each of 15 melanoma patients for total RNA extraction, reverse-transcription to cDNA, amplification, cloning, and transcription to produce unlimited amounts of copy mRNA.
Stabilizing unmodified mRNA by packaging in liposomes or forming complexes with cationic polymers has been widely investigated, as well as introducing chemical modifications to mRNA to make it more resistant against degradation and more efficient for translation. The latter includes elongation of the poly-A tail at the 3′-end of the molecule and modifications to the cap structure at the 5′-end. For example, if the original 7-methylguanosine triphosphate is replaced by an Antireverse Cap Analog (ARCA), the efficiency of transcription is strongly enhanced. To provide the immune system with even more potent signals, Scheel et al. modified mRNA with a phosphorothioate backbone in early commercial vaccine development work at CureVac GmbH (Tübingen, Germany) that continues today (see image below).
Effects of mRNA vaccines (taken from an article in Drug Discovery & Development by Ingmar Hoerr, PhD, CEO and Cofounder of CureVac).
In summary, in a 2013 review entitled RNA: The new revolution in nucleic acid vaccines, Geall et al. from Novartis Vaccines & Diagnostics (Cambridge, MA, USA) stated that “prospects for success are bright.” They site several reasons for this optimistic outlook including the potential of RNA vaccines to address safety and effectiveness issues sometimes associated with vaccines that are based on live attenuated viruses and recombinant viral vectors. In addition, methods to manufacture RNA vaccines are suitable as generic platforms and for rapid response, both of which will be very important for addressing newly emerging pathogens in a timely fashion. Plasmid DNA is the more widely studied form of nucleic acid vaccine and proof of principle in humans has been demonstrated, although no licensed human products have yet emerged. The RNA vaccine approach, based on mRNA, is gaining increased attention and several vaccines are under investigation for infectious diseases, cancer and allergy.
Modified mRNA for Expressing Clinically Beneficial Proteins
 Dr. Katalin Karikó, Adjunct Associate Professor of Neurosurgery and Senior Research Investigator, Department of Neurosurgery, University of Pennsylvania (taken from upenn.edu).
Dr. Katalin Karikó, Adjunct Associate Professor of Neurosurgery and Senior Research Investigator, Department of Neurosurgery, University of Pennsylvania (taken from upenn.edu).
In a landmark publication by Karikó et al. in 2008, it was reasoned that the suitability of mRNA as a direct source of therapeutic proteins in vivo required muting its immunogenicity and boosting its effectiveness. Clues as to how this might be achieved were provided in their earlier work demonstrating the use of base-modified triphosphates to enzymatically synthesize in vitro mRNA having modified nucleosides [such as, pseudouridine (Ψ), 5-methylcytidine (m5C), N6-methyladenosine (m6A), 5-methyluridine (m5U), or 2-thiouridine (s2U)>
had greatly diminished immunostimulatory properties. They reasoned that, “if any of the in vitro transcripts containing nucleoside modifications would remain translatable and also avoid immune activation in vivo, such an mRNA could be developed into a new therapeutic tool for both gene replacement and vaccination”.
Using the aforementioned and other base-modified nucleotide triphosphates—all obtained from TriLink BioTechnologies—Karikó et al. found, surprisingly, that mRNA containing pseudouridine had a higher translational capacity than unmodified mRNA when tested in mammalian cells and lysates or administered intravenously into mice at 0.015–0.15 mg/kg doses. The delivered mRNA and the encoded protein could be detected in the spleen at 1, 4, and 24 hours after the injection, and at each time-point there was more of the reporter protein when pseudouridine-containing mRNA was administered. Moreover, even at higher doses, only the unmodified mRNA was immunogenic. [Note: a fascinating follow-on publication provides a non-obvious—at least to me—molecular-level rationale for the surprising enhanced translation of pseudouridine-modified mRNA>
.
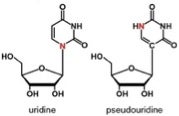 Uridine and pseudouridine differ in bonding to ribose but hydrogen-bond similarly to adenine. Pseudouridine is the most prevalent of the 100+ naturally occurring modified nucleosides found in RNA.
Uridine and pseudouridine differ in bonding to ribose but hydrogen-bond similarly to adenine. Pseudouridine is the most prevalent of the 100+ naturally occurring modified nucleosides found in RNA.
They concluded that, “[t>
hese collective findings are important steps in developing the therapeutic potential of mRNA, such as using modified mRNA as an alternative to conventional vaccination and as a means for expressing clinically beneficial proteins in vivo safely and effectively.” Prior to publishing this pivotal report, Katalin Karikó and co-author Drew Weissman filed a patent application in 2006 entitled RNA containing modified nucleosides and methods of use thereof that was issued on October 2, 2012 as US 8,278,036 and is assigned to the University of Pennsylvania.
Blood Boosting with Erythropoietin
 EPO stimulates the production of red blood cells (taken from proactiveinvestors.com via Bing Images)
EPO stimulates the production of red blood cells (taken from proactiveinvestors.com via Bing Images)
In a very persuasive demonstration of the real possibility of mRNA therapeutics, Karikó et al, reported in 2012 that non-immunogenic pseudouridine-modified mRNA encoding erythropoietin (EPO) was translated in mice and non-human primates. Indeed, a single injection of 100 ng (0.005 mg/kg) of HPLC-purified mRNA complexed to a delivery agent elevated serum EPO levels significantly and levels were maintained for 4 days. In comparison, mRNA containing uridine produced 10–100-fold lower levels of EPO lasting only 1 day. EPO translated from pseudouridine-mRNA was functional and caused a significant increase of both reticulocyte counts and hematocrits. As little as 10 ng mRNA doubled reticulocyte numbers. Weekly injection of 100 ng of EPO mRNA was sufficient to increase the hematocrit from 43 to 57%, which was maintained with continued treatment. Even when a large amount of pseudouridine-mRNA was injected, no inflammatory cytokines were detectable in plasma.
Using rhesus macaques (aka rhesus monkeys) they could also detect significantly-increased serum EPO levels following intraperitoneal injection of rhesus EPO mRNA. Other researchers (Kormann et al.) independently used a single injection of modified murine mRNA to produce EPO in mice.
Kick-Start Cardiac Repair with VEGF-A
That’s the catchy title of a News & Views article in the October 2013 issue of Nature Biotechnology with an equally catchy byline that reads “[t>
he survival of mice after experimental heart attack is greatly improved by a pulse of RNA therapy.” The featured report by Zangi et al., which is characterized as “a masterpiece of multidisciplinary studies…that will advance our thinking about therapeutic options in the cardiovascular arena,” is indeed impressive. These investigators report that intra-myocardial injections of vascular endothelial growth factor-A (VEGF-A) mRNA modified with 5-methylcytidine, pseudouridine, and 5’ cap structure resulted in expansion and directed differentiation of endogenous heart progenitors in a mouse model of myocardial infarction. They found markedly improved heart function and enhanced long-term survival of recipients. Moreover, “pulse-like” delivery of VEGF-A using modified mRNA was found to be superior to use of DNA vectors in vivo.
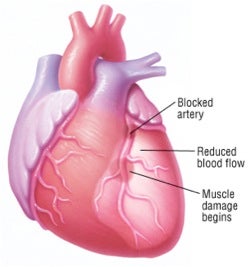 A heart attack (myocardial infarction) occurs when one of the heart's coronary arteries is blocked suddenly, usually by a blood clot (thrombus), which typically forms inside a coronary artery that already has been narrowed by atherosclerosis, a condition in which fatty deposits (plaques) build up along the inside walls of blood vessels (taken from drugs.com via Bing Images).
A heart attack (myocardial infarction) occurs when one of the heart's coronary arteries is blocked suddenly, usually by a blood clot (thrombus), which typically forms inside a coronary artery that already has been narrowed by atherosclerosis, a condition in which fatty deposits (plaques) build up along the inside walls of blood vessels (taken from drugs.com via Bing Images).
Notwithstanding these promising results, the aforementioned News & Views article points out that microgram-scale doses of modified mRNA in mice used by Zangi et al. “would probably correspond to several hundred milligrams…in humans delivered in volumes that might exceed 10 ml per heart. In clinical practice, it would be very difficult to administer such volumes to infarcted hearts.” In my humble opinion, these are legitimate but purely hypothetical issues at this time and, given that it’s very “early days” for therapeutic modified mRNA technologies, it’s not unreasonable to assume that new modifications and/or improved delivery strategies can be developed to enable clinical utility.
From a technical perspective, this work by Zangi et al. involves a form of cell-free reprogramming and, as such, is a good segue into the next section.
Modified mRNA for Cellular Reprogramming
In 2005, when I first heard of the concept of cellular reprogramming and dedifferentiation—which is to somehow coax a mature, differentiated cell to ‘run in reverse and go backwards biologically’ to a more primitive cell—my immediate impression as a chemist was this was impossible. Surely, I thought, this must violate the Second Law of Thermodynamics or, if not, is completely counterintuitive to how life works. Wow, was I wrong!
Reprogramming of differentiated cells to pluripotency is now firmly established and holds great promise as a tool for studying normal development. It also offers hope that patient-specific induced pluripotent stem cells (iPSCs) could be used to model disease or to generate clinically useful cell types for autologous therapies aimed at repairing deficits arising from injury, illness, and aging. Induction of pluripotency was originally reported by Takahashi & Yamanaka by enforced retroviral expression of four transcription factors, KLF4, c-MYC, OCT4, and SOX2 (aka “Yamanaka factors”)—collectively abbreviated as KMOS. (TriLink sells these and other factors used to direct cell fate.) Viral integration into the genome initially presented a formidable obstacle to therapeutic use of iPSCs. The search for ways to induce pluripotency without incurring genetic change has thus become the focus of intense research effort.
Consequently, much attention has been given to the 2010 publication by Warren et al. entitled Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. In this work complete substitution of either 5-methylcytidine for cytidine or pseudouridine for uridine in protein-encoding transcripts markedly improved protein expression, although the most significant improvement was seen when both modifications were used together. Transfection of modified mRNAs encoding the above mentioned Yamanaka factors led to robust expression and correct localization to the nucleus. Expression kinetics showed maximal protein expression 12 to 18 hours after transfection, followed by rapid turnover of these transcription factors. From this it was concluded that daily transfections would be required to maintain high levels of expression of the Yamanaka factors during long-term, multifactor reprogramming regimens.
They went on to demonstrate that repeated administration of modified mRNA encoding these (and other) factors led to reprogramming various types of differentiated human cells to pluripotency with conversion efficiencies and kinetics substantially superior to established viral protocols. Importantly, this simple, non-mutagenic, and highly controllable technology was shown to be applicable to a range of tissue-engineering tasks, exemplified by mRNA-mediated directed differentiation of mRNA-generated iPSCs to terminally differentiated myogenic (e.g. heart muscle) cells.
Modified mRNA reprogramming fibroblasts into induced pluripotent cells for directed differentiation into myofibers, according to Warren et al. in Cell Stem Cell (2010)
Warren et al. concluded that “we believe that our approach has the potential to become a major enabling technology for cell-based therapies and regenerative medicine.” According to the Acknowledgements section of this 2010 publication, corresponding author Derrick J. Rossi recently founded a company, ModeRNA [sic>
Therapeutics, dedicated to the clinical translation of this technology.” That, we shall see below, has had stunning commercial investment consequences.
By the way, and not surprisingly, Rossi & Warren filed a U.S. patent application in 2012 claiming, among other things, iPSCs induction kits using 5-methylcytidine- and pseudouridine-modified mRNA encoding KMOS human cellular reprogramming factors.
AstraZeneca’s Big Bet on Moderna’s Modified-mRNA Therapeutics
AstraZeneca aims to use Moderna Therapeutics’ modified-messenger RNA technology to develop and commercialize new drugs for cancer and serious cardiovascular, metabolic, and renal diseases, under a multi-year deal that could net Moderna more than $420 million. Moderna is also eligible for royalties on drug sales ranging from high single digits to low double digits per product.
AstraZeneca—ranked 7th in sales in 2010 among the world’s pharmaceutical companies—has the option to select up to 40 drug products for clinical development of what the companies are calling messenger RNA Therapeutics™, which could dramatically reduce the time and expense associated with creating therapeutic proteins using current recombinant technologies, the companies say. Moreover, “where current drug discovery technologies can target only a fraction of the disease-relevant proteins in the human genome, we have the potential to create completely new medicines to treat patients with serious cardiometabolic diseases and cancer,” AstraZeneca CEO Pascal Soriot said in a statement. Mr. Soriot, who had been a senior executive at Roche, very recently joined AstraZeneca, which said it would reorganize R&D and eliminate 1,600 jobs by 2016 as part of a plan to address issues related to failures in clinical trials of several drugs just as big sellers like the antipsychotic Seroquel and the heartburn drug Nexium have lost or are about to lose patent protection.
Moderna, based in Cambridge, Massachusetts, is privately held and was founded in 2010 by Flagship VentureLabs in association with leading scientists from Boston Children’s Hospital and Massachusetts Institute of Technology. Moderna has developed a broad intellectual property estate including 144 patent applications with 6,910 claims ranging from novel nucleotide chemistries to specific drug compositions, according to its website.
DARPA also Bets Big on Moderna’s Modified-mRNA Therapeutics
As the saying goes, “when it rains it pours”, and for Moderna it’s pouring money!
On October 2nd, Moderna announced that the U.S. Defense Advanced Research Projects Agency (DARPA)—whose most successful bets so far have been internet technologies—has awarded the company up to $25 million for R&D using its modified-mRNA therapeutics platform as a “rapid and reliable way to make antibody-producing drugs to protect against a wide range of now and emerging infectious diseases and engineered biological threats.” The statement goes on to say that Moderna’s approach can “tap directly into the body’s natural processes to produce antibodies without exposing people to a weakened or inactivated virus or pathogen, as in the case with the vaccine approaches currently being tested.”
The grant could support research for up to 5 years to advance promising antibody-producing drug candidates into preclinical testing and human clinical trial. The company also received a $700,000 ‘seeding’ grant from DARPA in March to begin work on the project.
If you’re interested in some of the possible ideas associated with the project, go to the 2013 patent application by Moderna entitled Methods of responding to a biothreat, which even envisages a portable, battery operated device for synthesizing modified mRNA. Oh well, never let it be said that DARPA fears a risky bet; on the other hand, since DARPA’s “playing with house money” (aka our taxes!), I suppose it’s easy for them. Let’s hope they/we all win.
Other Commercial Players
In addition to TriLink’s mRNA products, related services, and new cGMP facility, there are other companies to mention here, which I’ll do in alphabetical order.
- Acuitas Therapeutics has compared the effectiveness of its lipid nanoparticle (LNP) carriers in vivo with the most potent delivery systems reported in the scientific literature, and found that Acuitas LNPs demonstrate much greater luciferase expression in the liver after systemic administration.
- CureVac is combining both the antigenic and adjuvant properties of mRNA to develop novel and effective mRNA vaccines. CureVac is currently developing therapeutic mRNA vaccines in oncology and therapeutic/prophylactic vaccines for infectious diseases. Information on five of its clinical studies is available at ClinicalTrials.gov.
- Dendreon has a U.S. patent application for a method to make dendritic cell vaccines from embryonic stem cells that are genetically modified with mRNA encoding tumor antigen. However, no mRNA-searchable items are currently listed on Dendreon’s website.
- In-Cell-Art is investigating new and improved nanocarriers for mRNA vaccines, and has collaborated with Sanofi Pasteur and CureVac in DARPA-funded studies.
- Mirus Bio offers a TransIT®-mRNA Transfection Kit for high efficiency, low toxicity, mRNA transfection of mammalian cells, as described by Karikó et al.
Also noteworthy, the 1st International mRNA Health Conference recently held on October 23-24 at the University of Tübingen included talks by numerous key scientists in academia and industry that are well worth looking at in the Conference Program.
In conclusion, I hope that you found this emerging area of modified mRNA therapeutics as interesting and exciting as I did in researching this blog posting, and I welcome your comments.
Postscript
After finishing the above blog, I came across these additional publications on possible mRNA therapies.
Huang and coworkers reported earlier this year that systemic delivery of liposome-protamine-formulated modified mRNA encoding herpes simplex virus 1 thymidine kinase for targeted cancer gene therapy was significantly more effective than plasmid DNA in a therapeutic model of human lung carcinoma in xenograft-bearing nude mice.
Zimmermann et al. reported successful use of mRNA-nucleofection for overexpression of interleukin-10 in murine monocytes/macrophages for anti-inflammatory therapy in a murine model of autoimmune myocarditis. [Note: for a related report on mRNA-engineered mesenchymal stem cells for targeted delivery of interleukin-10 to sites of inflammation see Levy et al.>
Cystic Fibrosis (CF) is the most frequent lethal genetic disease in the Caucasian population. CF is caused by a defective gene coding for the cystic fibrosis transmembrane conductance regulator (CFTR). Bangel-Ruland et al. reported in vitro results indicating that CFTR-mRNA delivery provided a novel alternative for cystic fibrosis “gene therapy”.







