- In 2019, 1.7M People Were Newly Infected with HIV and 690,000 People Died from AIDS-related Illnesses.
- There Is No Vaccine to Prevent or Treat HIV Infection.
- This May Change with Use of Modified mRNA-Lipid Nanoparticles
Introduction
According to information from the National Institute for Allergies and Infectious Diseases (NIAID), for which Dr. Anthony Fauci serves as Director, HIV (human immunodeficiency virus) causes AIDS (acquired immunodeficiency syndrome) and can be transmitted during sexual intercourse, by sharing syringes, or perinatally during pregnancy, childbirth, or breastfeeding. First reported in 1981, HIV/AIDS has been one of humanity’s deadliest and most persistent epidemics. In the last 30 years, there have been nearly 400,000 publications related to HIV/AIDS, and these continue to rise, as shown by the chart here.
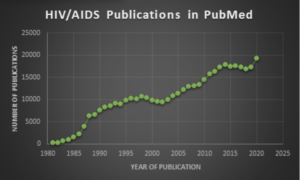 Search query (HIV[Title/Abstract>
Search query (HIV[Title/Abstract>
) OR (AIDS[Title/Abstract>
) and chart by Jerry Zon.
 US-supported HIV.gov states that there is currently no vaccine available that will prevent HIV infection or treat those who have it. It adds that “[d>
US-supported HIV.gov states that there is currently no vaccine available that will prevent HIV infection or treat those who have it. It adds that “[d>
eveloping safe, effective, and affordable vaccines that can prevent HIV infection is the NIH’s highest HIV research priority given its game-changing potential for controlling and ultimately ending the HIV/AIDS pandemic.” NIH-supported research efforts include two late-stage, multinational vaccine clinical trials called Imbokodo and Mosaico, both key components to the NIAID’s approach towards HIV vaccine development.
This blog will focus on some promising new results for an AIDS vaccine, which recently described by a large team of academic, medical, commercial, and NIAID collaborators in a bioRxiv preprint tilted Lipid nanoparticle encapsulated nucleoside-modified mRNA vaccines elicit polyfunctional HIV-1 antibodies comparable to proteins in nonhuman primates, referred to herein as Saunders et al.
As of February 2, 2021, this preprint has received more than 1,300 views following its initial posting on December 31, 2020, a testament to the interest in this work. The synopsis provided here is largely drawn from this preprint, which readers may consult for more detail.
Rationale
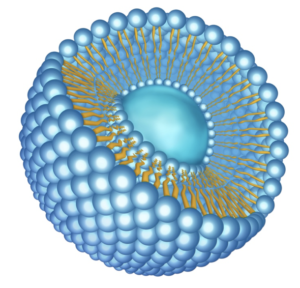 3D illustration of a simplified empty LNP structure.
3D illustration of a simplified empty LNP structure.
As reviewed elsewhere by Pardi et al., a number of technologies are currently being used to improve the pharmacological aspects of mRNA. These include 5’-capping, use of regulatory elements in the 5′-untranslated region (UTR) and the 3′-UTR to stabilize mRNA and increase protein translation, incorporation of chemically modified nucleosides into mRNA (modRNA) to decrease innate immune activation and increase translation, chromatographic purification, and sequence and/or codon optimization to increase translation. In addition, lipid nanoparticles (LNPs; illustrated here) loaded with immunogenic mRNA or modmRNA have proven effective for administration of vaccines, first for Zika virus in mice, and now for SARS-CoV-2 in humans.
mRNA/modRNA vaccines represent attractive options because they can be relatively rapidly designed against various pathogen antigens and manufactured at scale. This is unarguably evidenced by the unprecedented short amount of time (<1 year) it took to advance from sequencing SARS-CoV-2 to producing enough candidate modRNA/spike protein vaccine for large (30,000 volunteer) clinical trials, as well as hundreds of millions of doses of the two currently approved vaccines for COVID-19 by Pfizer/BioNTech (illustrated here) and Moderna.

All of the above factors, say Saunders et al., indicate that the modRNA-LNP platform could improve the elicitation of neutralizing antibodies against HIV (used herein interchangeably with HIV-1, the causative agent for AIDS). However, they add, little data exists on the comparative immunogenicity of these formulations and proteins in non-human primates, which are the animal model of choice for preclinical studies. Thus, according to Saunders et al., “a key question for HIV-1 vaccine development is whether the immunogenicity of mRNA vaccines encoding HIV-1 immunogens, a poorly immunogenic protein that requires extensive post-translational modifications, is comparable to that of proteins that can be purified after in vitro production.”
In this regard, HIV vaccines aiming to elicit protective antibody responses will likely need to elicit polyfunctional non-neutralizing effector antibodies (nnAbs) and/or, more importantly, broadly neutralizing antibodies (bnAbs), as discussed by Haynes et al. NnAbs are easy to induce and bind to the surface of virus-infected CD4+ T cells, where they can mediate antibody-dependent cellular cytotoxicity (ADCC). However, say Saunders et al., the efficacy of nnAbs in protecting against HIV transmission or controlling the disease in the setting of high transmission rates has been called into question.
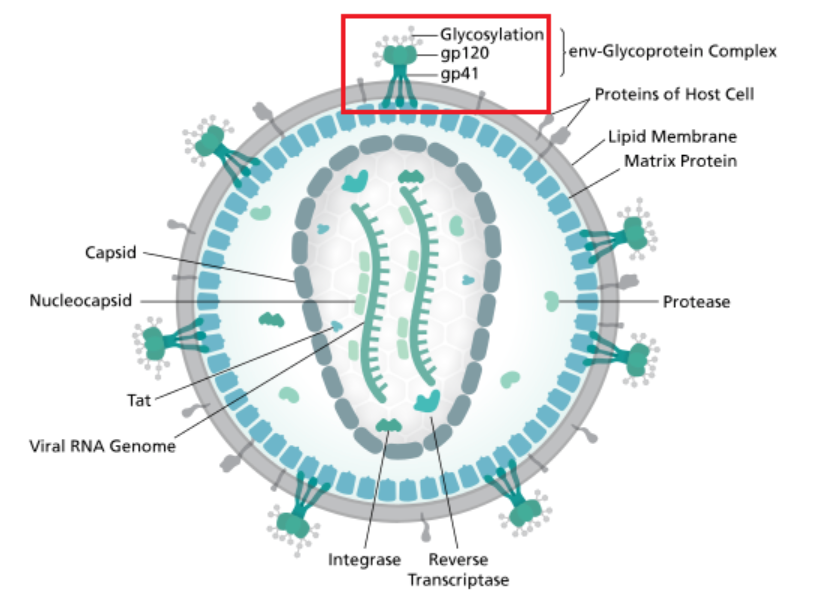
In contrast to nnAbs, Saunders et al. observe, bnAbs are difficult to induce for many reasons, including shielding of bnAb envelope (Env) epitopes by glycans (see HIV virion diagram caption), metastability of Env conformation, conformational masking of neutralizing epitopes, host immune controls preventing bnAb development, and the requirement of multiple highly improbable somatic mutations needed for acquisition of antibody neutralization breadth.
Finally, they conclude, mRNA immunization elicits antigen-specific follicular T helper cells (pictured here), which are key for affinity maturation of antibodies in germinal centers, and have been shown to correlate with bnAb development during natural infection. Regardless of whether nnAbs or bnAbs are targeted, the poor durability of HIV-1 Env antibody and the necessity for a vaccine that has multiple components for bnAb induction necessitates novel vaccine platforms that can maintain protective antibody levels.
Saunders et al. therefore compared the antibody responses in rhesus macaques (pictured here) induced by either HIV Env-encoded as modRNA-LNP, or the same vaccine candidate administered as an adjuvanted HIV Env recombinant protein. As summarized in the next section, when compared to protein immunization, modRNA-LNP immunization elicited either the same or superior magnitude and breadth of HIV Env-specific polyfunctional antibodies. Doses of HIV Env gp160 modRNA-LNP as low as 5 μg were immunogenic in macaques and, importantly, induced durable neutralizing antibodies for approximately one year.
Methods
In keeping with the nucleic acids-related scope of the Zone, the emphasis will be on the preparation of key materials for testing, namely, the modRNA-LNP formulations. Details on these and additional methods used can be found in Saunders et al., which is entirely free and available with Supplementary Material.
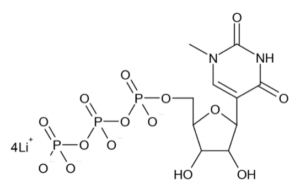
ModRNA production: As previously described, the various modRNAs were produced using T7 RNA polymerase on linearized plasmids encoding codon-optimized immunogens, including several variants for each gp160 HIV-1 Env, chimeric stabilized HIV-1 Envs, and gp120 HIV-1 Envs. The mRNAs were transcribed to contain 101 nucleotide-long poly(A) tails, based on the DNA-encoded poly(A) tail sequence. The modified nucleoside analog N1-methylpseudouridine (m1Ψ)-5’-triphosphate (depicted here), which was obtained from TriLink, replaced uridine-5’-triphosphate (UTP) during in vitro transcription (IVT). The resultant modRNAs were enzymatically capped as a type 1 cap, which can be much more efficiently incorporated by IVT with TriLink’s co-transcriptional CleanCap® Analog [see Henderson et al. in Current Protocols (2021)>
. Capped modRNAs were purified by Fast Protein Liquid Chromatography (FPLC) as previously described, and were analyzed by denaturing or native agarose gel electrophoresis before being stored frozen at −20°C.
LNP formulation of modRNA: FPLC-purified m1Ψ-containing HIV-1 Env modRNAs were encapsulated in LNPs using a self-assembly process, in which an aqueous solution of modRNA at pH=4.0 was rapidly mixed with a solution of lipids dissolved in ethanol. The LNPs were similar in composition to those previously described: biodegradable lipids enabling rapidly eliminated LNPs for systemic delivery of RNAi therapeutics, which contained an ionizable cationic lipid (US patent US10,221,127) /phosphatidylcholine/cholesterol/PEG-lipid, and had a diameter of ~80 nm.
Results
Saunders et al. carried out numerous experiments, the results for which far exceed the scope of this blog. Consequently, the Zone has selected results that provide an indication of key findings. Readers interested in full details are encouraged to consult the original preprint.

Protein and modRNA vaccinations each lead to high titers of HIV-binding antibodies. For non-immunologists: antibody titer is a measurement of how much antibody that recognizes a particular epitope is produced, expressed as the inverse of the greatest dilution (in a serial dilution) that still gives a positive result. The enzyme-linked immunosorbent assay (ELISA) is a common means of determining antibody titers, as exemplified here for a typical colorimetric microwell plate-based readout.
Saunders et al. compared the immunogenicity of HIV-1 Env A244 gp120 lacking the first 11 N-terminal amino acids of gp120 (Δ11 gp120, as in the RV144 trial), administered as either modRNA-LNP or adjuvanted recombinant protein in two groups of five macaques. One group received A244 Δ11 gp120, formulated with a common aluminum hydroxide adjuvant (Rehydragel). Adjuvants vary in their immunostimulatory strength, and in a second group, the immunogenicity of the A244 Δ11 gp120 Env was evaluated in combination with a known liposomal adjuvant (ALFQ). Additionally, two groups of macaques were immunized with nonadjuvanted modRNA-LNP encoding either A244 Δ11 gp120 or A244 Δ11 gp120, with a D368R mutation that disrupted the CD4 binding site. This was done to determine whether Env binding to CD4+ T cells in vivo altered immunogenicity of HIV-1 Env gp120. Macaques were immunized with either protein or modRNA-LNP at weeks 0 and 6, and antibody responses were followed for 18 total weeks.
Comparable neutralizing and non-neutralizing antibodies induced by adjuvanted Env gp120 protein vs. mRNA-LNP immunization: Plasma IgG from all four groups of macaques was able to bind HIV-infected cells, as quantified by mean fluorescence intensity of bound IgG or the percentage of cells positive for HIV-1 protein p24 and plasma IgG. Results for modRNA-LNP vaccination and protein adjuvanted with ALFQ were not different and, in agreement with the overall lower IgG titers, Rehydragel-adjuvanted protein gave the weakest cell-binding responses.
ADCC titers were approximately one order of magnitude higher when ALFQ was used as the adjuvant instead of Rehydragel. Similarly, mRNA-LNP immunization induced activity lower than ALFQ-formulated protein, but 3-fold higher than protein adjuvanted with Rehydragel. Elimination of CD4 binding to A244 Δ11 gp120 had no effect on ADCC activity. Saunders et al. also measured antibody-dependent cellular phagocytosis (ADCP). This provides mechanisms for clearance of virus and virus-infected cells (illustrated here), as well as for stimulation of downstream adaptive immune responses by either facilitating antigen presentation or stimulating secretion of inflammatory mediators. Median plasma ADCP activity against A244 gp120-coated beads was similar across all groups, with A244 Δ11 gp120 in Rehydragel eliciting a wider range of responses. In agreement with previous studies, modRNA-LNP vaccination elicited antibody effector functions that have been previously shown to correlate with reduced infection risk.
While the goal of A244 Δ11 gp120 immunization was to induce non-neutralizing effector functions like those seen in the RV144 trial, Saunders et al. compared the elicitation of neutralizing antibodies of each vaccine. They found that there was no significant difference among the different vaccination regimens for induction of HIV neutralizing antibodies.
ModRNA-LNP immunization induces durable antibody responses against HIV-1 Env: According to Saunders et al., inducing durable Env antibody responses is a key goal of HIV vaccine development. However, in the RV144 trial, protective antibodies fell dramatically over the first 42 weeks after vaccination. Thus, they say, if immunization with modRNA-LNP induced durable antibody responses, it would benefit their use as a vaccine platform for many different infectious diseases.
The durability of antibody responses was assessed using neutralization assays of a particular HIV type, namely tier 1 CH505 w4.3 virus, which was chosen for reasons detailed by Saunders et al. Longitudinal comparative analyses of neutralization of the CH505 w4.3 virus was carried out with modRNA-LNP encoding gp160 (see caption in the above diagram of the HIV virion), which elicited higher titers of neutralizing antibodies than the modRNA-LNP encoding gp140 SOSIP trimer, a soluble and stabilized version of the functional envelope spike. Notably, neutralization of the CH505 w4.3 virus was still detectable in all three groups 12 weeks after the last immunization, although with a downward trend that raised the question of how long the neutralizing antibodies would persist at detectable levels.
Discussion
Saunders et al. offered multiple factors with supporting literature references to explain why modRNA immunization has potent immunogenicity for viral proteins. First, modRNA is delivered by LNPs to antigen-presenting dendritic cells, and likely to other immune cells that are able to activate naïve T cells to respond to the vaccine immunogen. Second, modRNA-LNP immunization generates robust antigen-specific germinal center T and B cell responses, as depicted here. Within the germinal center, follicular T helper cells provide help to B cells undergoing affinity maturation. The affinity maturation process is critical for the development of high-affinity antibodies after vaccination, and is particularly required for HIV-1 bnAb development.
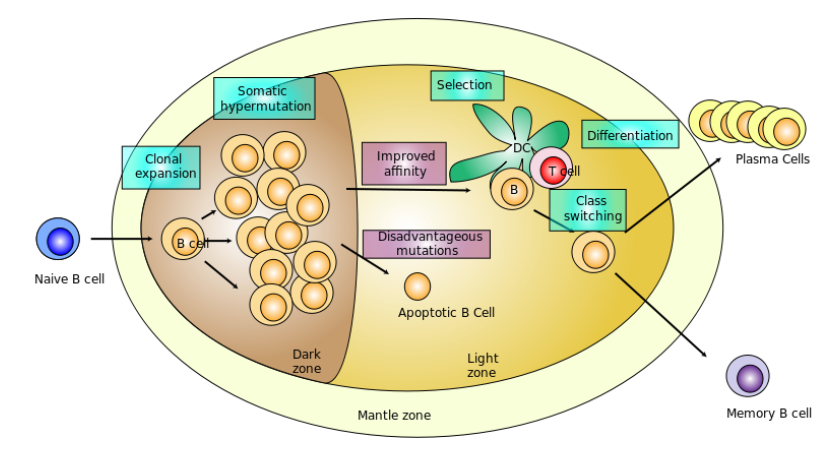
Animal models vaccinated with modRNA-LNPs encoding HIV-1 Env develop high levels of pathogen-specific T follicular helper cells. Given that HIV-1 neutralizing antibody breadth is correlated with the frequency of circulating T follicular helper cells, modRNA-LNP immunization warrants further study as an HIV-1 vaccine platform. Moreover, modRNAs have many potential advantages over proteins, including speed of production, cost-savings, and compatibility with current good manufacturing practices (cGMPs) for the multicomponent vaccines that are needed to induce both protective HIV-1 bnAbs and nnAbs.
In summary, say Saunders et al., “comparison of modRNA-LNPs with recombinant Env proteins in three adjuvants demonstrated the utility of modRNA-LNPs as a mode of inducing nnAbs that are predicted to be protective against retrovirus challenge. We suggest that moving to modRNA-LNPs for the next generation of clinical trials for multivalent Env immunization will be advantageous and speed the development of a globally available protective HIV-1 vaccine.”
Concluding Comments
The Zone is very impressed by the promising findings of Saunders et al. for modRNA-LNPs as a platform to develop an effective vaccine for AIDS, which remains a pressing unmet medical need.
What do you think?
Your comments are welcomed, as usual.
Please feel free to share this blog with your colleagues or on social media.