As we enter October, national breast cancer awareness month, I thought it an appropriate time to review some of the latest advances in breast cancer research. Let’s start with a review of the latest statistics published by the NCI and a look at current treatments, then we’ll explore some of the most promising new research in this field.
According to the National Institutes of Health (NIH) National Cancer Institute (NCI) Cancer Topics for Breast Cancer, the projected statistics on breast cancer for women in the United States in 2013 are grim: 232,340 new cases and 39,620 deaths. Moreover, global statistics for breast cancer were estimated to be over 1.5 million new diagnoses and 500,000 deaths in 2010—with the majority of these being in low and middle income countries, which presents a major challenge of a different kind.
The NIH NCI website provides an informative online booklet entitled What You Need To Know About™ Breast Cancer to learn about breast cancer types, staging, treatment, and questions to ask the doctor. There is also NCI’s overview of Cancer Advances in Focus: Breast Cancer that provides the following perspectives for “today” and “tomorrow.”
Today
- Among women diagnosed with breast cancer during the period from 1999 through 2006, 90% were expected to survive their disease at least 5 years. Among white women, the 5-year relative survival rate was 91%; among African American women, it was 78%. The increase in breast cancer survival seen since the mid-1970s has been attributed to both screening and improved treatment.
- Breast-conserving surgery (lumpectomy) followed by local radiation therapy has replaced mastectomy as the preferred surgical approach for treating early-stage breast cancer.
- Routine mammographic screening is an accepted standard for the early detection of breast cancer. The results of eight randomized trials, the NIH-ACS Breast Cancer Detection Demonstration Projects, and other research studies showed that mammographic screening can reduce the mortality from breast cancer.
- Hormonal therapy with selective estrogen receptor modulators (SERMs), such as tamoxifen, and aromatase inhibitors is now standard in the treatment of women with estrogen receptor-positive breast cancer, both as adjuvant therapy and in the treatment of advanced disease. Estrogen receptor-positive breast cancer cells can be stimulated to grow by the hormone estrogen. SERMs interfere with this growth stimulation by preventing estrogen from binding to the estrogen receptor. In contrast, aromatase inhibitors block estrogen production by the body. Food and Drug Administration (FDA)-approved aromatase inhibitors include anastrozole, exemestane, and letrozole.
- Tamoxifen and another SERM, raloxifene, have been approved by the FDA as treatments to reduce the risk of breast cancer in women who have an increased risk of developing the disease.
- The monoclonal antibody trastuzumab is an accepted treatment for breast cancers that overproduce a protein called human epidermal growth factor receptor 2, or HER2. This protein is produced in abnormally high amounts by about 20% of breast tumors. Breast cancers that overproduce HER2 tend to be more aggressive and are more likely to recur. Trastuzumab targets the HER2 protein specifically, and this antibody, in conjunction with adjuvant chemotherapy, can lower the risk of recurrence of HER2-overproducing breast cancers by about 50% in comparison with chemotherapy alone.
- Several breast cancer susceptibility genes have now been identified, including BRCA1, BRCA2, TP53, and PTEN/MMAC1. Approximately 60% of women with an inherited mutation in BRCA1 or BRCA2 will develop breast cancer sometime during their lives, compared with about 12% of women in the general population.
Tomorrow
- We will use our rapidly increasing knowledge in the fields of cancer genomics and cell biology to develop more effective and less toxic treatments for breast cancer and to improve our ability to identify cancers that are more likely to recur. Moreover, we will use this knowledge to tailor breast cancer therapy to the individual patient.
- We will use our increasing knowledge of the immune system to enhance the body’s ability to recognize and destroy cancer cells. The knowledge we have acquired thus far has facilitated the development of several promising breast cancer treatment vaccines that are currently under clinical evaluation.
- We will use advanced technologies, including genomic technologies, to improve our ability to detect breast cancer at its earliest stages, when it is most treatable, and to better define individual risk for this disease.
- We will strive to understand, address, and eliminate factors that contribute to the higher mortality from breast cancer experienced by African American women compared with women of other racial and ethnic groups.
Managing the Nation’s Cancer Research Portfolio
As a scientist and taxpayer, you’ll likely be very interested—as I was—in knowing much more about the specifics of what the NCI proposed for managing the Nation’s cancer research portfolio in 2013. This information is nicely laid out at an NCI website that includes a downloadable pdf version with programmatic details and a summary budget ($5.833 Billion).
Among the details that I found particularly interesting is an introductory section called “Provocative Questions.” NCI launched an initiative late in 2010, seeking to go beyond the questions that are self-evident or that have been studied for many years. NCI “asked investigators to propose intriguing questions that need attention but might not otherwise get it or that have stumped us in the past but may be answered by new technologies. The initiative, which elicited a strong and exciting response from the research community, has recently funded its first 56 investigators.” In addition to a link therein for the Provocative Questions Project website, there is a link to a short video wherein NCI Director Harold Varmus, MD, discusses this project.
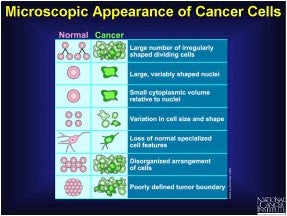
How do Normal v. Breast Cancer Cells Appear Microscopically?
From the descriptors given in the summary slide, provided by NCI, it is evident that breast cancer cell shape, size, and other properties reflect cellular dysfunctions, relative to normal cells, as is generally characteristic for other types of cancer. The accompanying high-resolution color enhanced scanning electron micrograph (SEM) of a breast cancer cell is visually stunning yet scary in view of the aforementioned death rate statistics.
Spotlight on an Expert
Prof. Esther H. Chang, MD PhD, is a member of the Departments of Oncology and Otolaryngology at the Lombardi Comprehensive Cancer Center of Georgetown University Medical Center. Before joining Georgetown University, Dr. Chang held positions at the National Cancer Institute (NCI), Stanford University, and the Uniformed Services University of Health Sciences. Currently, she is serving as the Interim President of the American Society for Nanomedicine and she is also an Executive Board Member of the International Society for Nanomedicine (ISNM) in Basel. Dr. Chang is the founding scientist of, as well as a Senior Consultant for, SynerGene Therapeutics, Inc. She has over 130 publications and has served as a member of a number of scientific advisory boards for NCI, NASA, the US Military Cancer Institute, and the Department of Energy.
I had the good fortune to become a collaborator of Dr. Esther H. Chang when she was working at the Uniformed Services University of Health Sciences, and I was literally across the street working at the FDA at NIH. We found that we shared a common interest in antisense therapeutics, which at that time (1980s) was a relatively new and—importantly—entirely novel drug development paradigm envisaged as affording more specific, less toxic anticancer agents. My lab could synthesize various chemically modified oligos, but had no molecular or cellular biology expertise. Dr. Chang’s lab at the time was working on Ras, which is a common oncogene in human cancer. We eventually published those initial collaborative investigations, and later reported a series of three papers on tumor-specific targeting and delivery of modified hybrid (DNA-RNA) anti-HER-2 siRNA analogs developed by TriLink (click here for details).
Dr. Chang’s achievements in specifically targeting tumors using antibody-fragment-tagged liposomal nanoparticles are indeed notable—and quotable—as outlined below.
"Tiny little FedEx trucks"
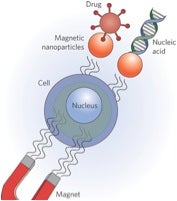
As scientists, we can appreciate the need to occasionally over simplify very complex technical concepts, and scientific jargon, in order to talk to nonscientists about our research. Consequently, I had to smile with appreciation when Dr. Chang referred to tumor cell-specific, targeted delivery of liposomal nanoparticles as involving “tiny little FedEx trucks” when interviewed on National Public Radio that can be listened to by clicking here.
Dr. Chang’s use of this catchy metaphor is apropos for a couple of reasons. Just as trucks carry various types of cargo, liposomal nanoparticles (spheres pictured) can be loaded with various classes of therapeutic agents, ranging from traditional small-molecule entities to high-molecular weight genes, mRNAs, and mi/siRNA. Also, just as FedEx employs advanced technologies to locate ZIP-code intended-recipients, nanoparticles can utilize antibodies, fragments of antibodies (Y-shaped molecules pictured above) or other agents (ligands) for specific delivery to intended target cells via binding to cell surface receptors (pink molecules pictured above).
In an early publication entitled Tumor-targeted p53-gene therapy enhances the efficacy of conventional chemo/radiotherapy, Dr. Chang noted that a long-standing goal in gene therapy for cancer is a stable, low toxic, systemic gene delivery system that selectively targets tumor cells, including metastatic disease. In particular, ligand-directed tumor targeting of cationic liposome-DNA complexes (lipoplexes) showed promise for targeted gene delivery and systemic gene therapy. She demonstrated that Lipoplexes developed in her lab directed by ligands such as folate, transferrin or anti-transferrin receptor scFv antibody fragment, showed tumor-targeted gene delivery, expression, and anti-cancer effect in mouse models of human breast cancer, as well as prostate, head and neck cancers, seeming to meet these goals.
Based upon these promising results, this nanodelivery system has moved in human clinical trials. When I asked Dr. Chang about the status of such trials, and whether these results were applicable is some way to breast cancer, she offered the following comments:
“Tumor specificity is very crucial to a cancer therapy’s efficacy,” Chang asserts. “Because we have a targeting moiety, the nanocomplex will travel through the bloodstream and whenever it encounters a tumor cell — whether a primary tumor or metastasis — the nanoparticle will bind to it, enter the tumor cell and destroy it.”
This tumor targeted nanomedicine delivering the p53 gene (SGT-53) has already completed a Phase I safety trial, the results of which were described in an article entitled Phase I Study of a Systemically Delivered p53 Nanoparticle in Advanced Solid Tumors published in May of this year. The purpose of this trial was to give back to cancer patients the tumor-busting p53 gene they have lost.
She continued, “We were very encouraged by the results since the patients handled SGT-53 very well. Only minimal side effects occurred. Even more exciting, at the end of treatment the majority of patients showed at least stable disease. We were also able to demonstrate targeted delivery of SGT-53 to metastatic cancer, and not to normal cells. These agents are intended to increase the effects of standard therapies by sensitizing the tumor cells to their killing effects. Thus, cancer is less likely to recur. We have seen fantastic anti-cancer responses in animal models with breast cancer, and we have seen this treatment working in various types of solid tumors in patients. Therefore, we think it is very likely that it will also be successful in treating breast cancer.”
Regarding future development of this agent, Chang stated “Three Phase II clinical trials are imminent in patients with pancreatic cancer, glioblastoma and lung cancer. We hope to evaluate this agent in patients with breast cancer in the near future.”
More on Nanomedicine for Cancer Therapy
Researchers in nanomedicine (a term first used in the late 1990s) aim to develop a variety of revolutionary tools:
- Drug carriers that focus drug action at the site of disease to limit side effects.
- Vaccines that are more feasible for use in areas with limited health care access (more stable, less expensive, and with minimal adverse reactions).
- Imaging agents that produce signal detectable from significant depths only in diseased tissues.
- Scaffolds for culturing engineered tissues that mimic the corresponding extracellular matrix and enable modulation of development over time and real-time monitoring of their activity and biochemistry.
As with any scientific field, its boundaries blur with those of related fields, including pharmaceutics, bio- and materials engineering, nuclear medicine, tissue engineering, and many others.
Magnetically Triggered, Local On-Demand Release of Drugs
Tran & Wilson highlight a particularly fascinating approach in nanomedicine aimed at making controllable magnetic drug delivery possible for the treatment of breast cancer. They note that a recent study published by Kong et al. in Nano Letters documents the synthesis and performance of porous silica nanocapsules filled with magnetic nanoparticles as a controllable magnetic drug delivery vector. Under a remotely applied radiofrequency magnetic field, these nanocapsules demonstrate on-off switchable release of the internally loaded drug payload. Both in vitro and in vivo studies using mouse breast cancer cell models demonstrate that the magnetic targeting of these nanocapsules allows for deep tumor penetration and subsequent on-demand release of the drug cargo, significantly reducing tumor cell viability.
"Chemical Antibodies" - Improving Nature with RNA Aptamers
Earlier this year, Shigdar et al. reported the use of sensitive “chemical antibodies” for diagnosis in breast cancer. They state that Epithelial cell adhesion molecule (EpCAM) is expressed at low levels in a variety of normal human epithelial tissues, but is overexpressed in 70–90% of carcinomas. From a clinico-pathological point of view, this has both prognostic and therapeutic significance. EpCAM was first suggested as a therapeutic target for the treatment of epithelial cancers in the 1990s. However, following several immunotherapy trials, the results have been mixed. It has been suggested that this is due, at least in part, to an unknown level of EpCAM expression in the tumors being targeted. Thus, selection of patients who would benefit from EpCAM immunotherapy by determining EpCAM status in the tumor biopsies is currently undergoing vigorous evaluation. However, current EpCAM antibodies are not robust enough to be able to detect EpCAM expression in all pathological tissues. Shigdar et al. go on to report a newly developed EpCAM RNA aptamer, also known as a chemical antibody, which is not only specific but also more sensitive than current antibodies for the detection of EpCAM in formalin-fixed paraffin-embedded (FFPE) primary breast cancers.
Schematic of dye- or drug-containing nanoparticle targeted to cancer cells by Y-shaped RNA aptamers (taken from Chemical & Engineering News 2012 via Bing Images)
Twenty-mer aptamers were chemically synthesized with 2′-fluoro- or 2′-O-methyl-pyrimidine bases, a 3′-inverted deoxythymidine, and 5′-fluorescent tags. One of these aptamers showed no non-specific staining or cross-reactivity with tissues that do not express EpCAM. They were able to reliably detect target proteins in breast cancer xenograft where an anti-EpCAM antibody showed limited or no reactivity. They conclude that these results show the potential of aptamers in the future of histopathological diagnosis and as a tool to guide targeted immunotherapy.
I hope you leave this blog inspired by the promising developments that have been made in the field of breast cancer research and optimistic about the strides we, as a research community, continue to make toward finding a cure.
Breaking News: FDA backs Roche drug as first-of-a-kind therapy to treat breast cancer before surgery
On September 12th, the Washington Post reported online that the FDA’s panel of cancer experts voted 13-0, with one abstention, that the benefits of Perjeta (a monoclonal antibody developed at Genentech) as an initial treatment for breast cancer outweigh its risks. The recommendation is not binding, but sets the stage for the FDA to clear the drug as the first pharmaceutical option approved to shrink or eliminate tumors before surgery. “We are supporting the movement of a highly active drug for metastatic breast cancer to the first-line setting, with the hope that women with earlier stages of breast cancer will live longer and better,” said Dr. Mikkael Sekeres, an associate professor of medicine at the Cleveland Clinic. Doctors hope that using cancer drugs earlier could help shrink tumors, making them easier to remove. In some cases, that could allow women to keep their breasts, rather than having a full mastectomy.