Nucleic acids are getting small, SMARTT, long and weird
Like the “kid in the candy store,” it was a challenge for me to decide which of the many scheduled talks on oligonucleotide therapeutics and nucleic acids diagnostics at the 15th Anniversary TIDES 2013 conference would provide “tasty” blog content. Even more challenging was how to get that content since I couldn’t be at this event in Boston on May 12-15! Continuing the metaphor, “candy” I eventually selected scientific diversity to hopefully satisfy the different ”tastes” of readers.
The solution for how to be there, yet not be there, had me stumped. Fortunately my cleaver colleagues at TriLink got me there as a life-size cardboard standup for a “Bit of Boston” contest (check out all the pictures of me and TIDES attendees in my May 12 post), while Rick Hogrefe (President/CEO) offered to be my eyes and ears, so to speak. Following is Rick’s report to which I’ve added some visuals, links, and comments (in italics).
Getting Small: Spherical Nucleic Acids Nanoparticle Delivery
Prof. Chad A. Mirkin, Northwestern University and AuraSense Founder
JZ Comments: Prof. Mirkin’s outstanding scientific accomplishments, pioneering nanotechnology research, and list of numerous awards - including the 2013 Linus Pauling Medal Award, recipients of which have frequently (~50%) gone on to receive a Nobel Prize - may be found at the Mirkin Research Group website.
 Prof. Chad A. Mirkin - the most cited chemist in the world (past decade, Thomson Reuters) and the most cited nanomedicine researcher in the world.
Prof. Chad A. Mirkin - the most cited chemist in the world (past decade, Thomson Reuters) and the most cited nanomedicine researcher in the world.
The normal course of drug discovery usually starts with a drug concept in search of a discovery vehicle. Rarely does a discovery vehicle create a new drug concept with its own set of remarkable properties. Such may have been the luck of researchers at the International Institute for Nanotechnology directed by Prof. Mirkin - a man of many talents and head of a research group larger than many biopharmaceutical companies - who has led a nearly two-decade effort delving into the creation and use of nanoparticles combined with oligonucleotides. His earlier collaborative work, with many others at Northwestern, has led to commercial successes in the diagnostic world and nanofabrication (see Nanosphere and NanoInk for details). However, recent discoveries regarding therapeutic applications of oligonucleotide-functionalized nanoparticles has led to what may be the development of an entirely new class of therapeutic nucleic acids called spherical nucleic acids (SNA).
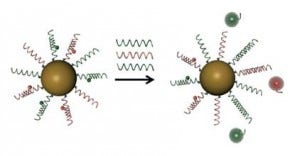
As depicted above, SNA are comprised of an inorganic (e.g., gold, silver, silica, etc.) inner core and a nucleic acid outer shell. These compact, 13 nm particles exhibit unusual hybridization properties that are largely based on the cooperativity of densely packed strands and high local concentrations. Transitions from duplex to single strands that normally occur over a 20°C range take place in a 2-8°C range on SNA.
This property was exploited as a diagnostic tool by preparing fluorescent probes Mirkin called “flares” (aka nano-flares) that are hybridized to the oligonucleotides on the gold SNA and thereby quenched. Because of the very sharp melting transition, they are able to detect with greater accuracy a wider range of sequences compared to existing microarray technologies. When the SNA oligonucleotides hybridize to the correct target, the tagged probes are released and light up, like a flare, due to unquenching of the fluorophore. The SNA wiki site provides many interesting details about SNA structure, function, applications and societal benefits of this novel class of materials, including gene regulation, molecular diagnostics, intracellular probes, and materials synthesis.
Mirkin also reported that SNA have a range of other favorable properties useful for a therapeutic. SNA-bound oligonucleotides are nuclease resistant and very non-immunostimulatory in comparison to other delivery vehicles. Perhaps the most remarkable property is the ability for SNA to permeate almost any cell and distribute to most tissues including the brain. Approximately 1% of the material administered to an animal crosses the blood brain barrier. They demonstrated the ability of an SNA to reduce the level of Bcl2L12 expression in a mouse glioblastoma model using systemic delivery. Read more about this line of research on anti-glioma therapeutics by the Mirkin group using novel siRNA-conjugated gold nanoparticles.
The mechanism of delivery was explored at length, with the most likely mode being uptake through caveolae-mediated endocytosis. Class A scavenger membrane receptors, which are known to bind various polyanionic ligands that include polynucleotides, were found to likely be associated with this active transport. Down-regulation of class A scavenger receptors resulted in cessation of uptake. Also revealing was the fact that the nanoparticles required a nucleic acid shell in order to be taken up. Controls using other conjugates were not taken up by cells. In my conversation with Dr. Sergei Gryaznov - recently appointed as CTO of AuraSense Therapeutics, a subsidiary of AuraSense that commercializes SNA - he speculated that perhaps a high density of anionic ligands is all that is required in order to achieve uptake.
Regardless of which applications of SNA meet with success, it’s clear that Prof. Mirkin and his team have done a remarkable job at both developing and commercializing a very interesting class of nanoparticles that we will definitely keep an eye on.
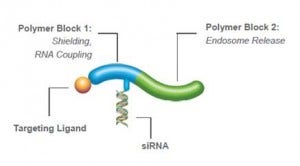
Getting SMARTT: Block-Polymer Delivery
Dr. Mary Prieve, PhaseRx
PhaseRx, located in Seattle, entered the nucleic acid delivery fray in 2008. Their lead technology, called the SMARTT Polymer, is a vinyl polymer delivery system that consists of three domains. The first is the targeting portion of the molecule. The second is the payload carrier, to which the nucleic acid is conjugated in the examples shown. The third is designed to facilitate release from the endosome, which is the mode of uptake of the particle.
PhaseRx proof-of-concept studies have focused on hepatocellular cancer (HCC). Two genes, MET, a cell surface receptor, and CTNNB1 (β-catenin), a cell adhesion protein, are often found to be overexpressed in many HCC patients. siRNA sequences were found that specifically down regulated those genes. PhaseRx conducted a very eloquent animal study wherein they introduced the MET and CTNNB1 genes into mice using plasmids, demonstrating that co-expression of these genes can induce HCC, and that they can subsequently turn off the effect using their formulated siRNAs. The result was a significant reduction in liver tumor size and alpha-fetoprotein (AFP) levels. They then modified the MET gene such that the gene still functioned to induce HCC, but did not match the siRNA sequence developed for wild type MET. They obtained the result they had hoped for: the modified MET gene was completely uneffected by the complex.
The audience had several interesting questions, one being whether this vinyl polymer is biodegradable. Dr. Prieve said those studies are currently ongoing. Another question was about the payload and the number of siRNAs conjugated to each particle, to which the answer was one per particle. According to PhaseRx’s website, it is “deploying an integrated delivery system based on novel synthetic polymers that delivers RNAi drugs into the cytoplasm by mediating their escape from intracellular vesicles called endosomes, where these drugs are often trapped and sequestered. The synthetic polymers exploit natural cellular processes and pH changes to effect endosome escape, delivering RNAi drugs into the cytoplasm where they can reach and inhibit the desired target of interest. The PhaseRx system offers significant advantages: effective intracellular RNAi delivery, broad applicability, and consistency with pharmaceutical development standards.”
Getting Long: Targeting Long Non-Coding RNA
Dr. Jim Barsoum, CSO, RaNA Therapeutics
RaNA Therapeutics is focusing on the latest in the RNA craze, long non-coding RNA (lncRNA). The presentation started with a much needed beginners course on lncRNA and what are some of its functions (Wikipedia has some good resources as well if you’re interested). Naturally, new findings will most likely indicate that much of these proposed functions are completely wrong, and that lncRNA operates in some other fashion, but that RaNA’s core technology still works (read on…). Call that the cynicism of someone who follows the slow unveiling of the roles of microRNAs in the cell. RNA biology is perhaps at one of its most exciting times. The range of tools available today for the RNA scientist from commercial sources is remarkable, and “RNA-ologists” are exploiting these tools to make great strides forward.
One new fact that I took home is that lncRNA is often 5’ capped and 3’ polyadenylated, just like mRNA, however they are very poorly expressed, if at all. The reason for this was not given. One possibility that came to my mind was that perhaps lncRNAs are degraded by the same mechanism as mRNA. Another possibility is that they require circularization to function using the same proteins that bind 5’ cap to the 3’ poly-A tail that is required by mRNA in order to be translated. Another interesting fact is that of all the RNA that is transcribed, only ~1% is mRNA for the production of protein. There is a lot of unknown science in the other ~99%.
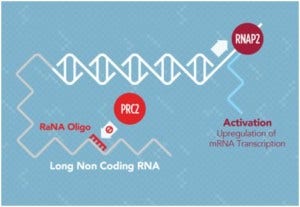
JZ Comments: To put Rick’s further remarks in context, I’ve included the mechanism from RaNA’s website for long noncoding RNA. The description for the mechanism says, “RaNA’s proprietary technology upregulates the expression of desirable genes that can prevent or treat disease. RaNA’s approach operates epigenetically and reverses the endogenous repression of gene expression. RaNA’s therapeutic oligonucleotide can be administered as a subcutaneous injection in saline, and is then taken up by cells in most tissues of the body, crossing the endosome membrane to enter the cytoplasm and nucleus. When transcription of the unique lncRNA target reveals the PRC2 binding domain the RaNA antagonist therapy binds the lncRNA, which blocks PRC2 recruitment and allows for transcription to proceed resulting in mRNA upregulation.”
RaNA is exploiting one function of lncRNA which is the control of transcription in a cell. The lncRNA silences genes by recruiting transcriptional repressor proteins, such as polycomb repressor 2 (PRC2) by binding to the target gene in a standard hybridization mode with part of lncRNA and to the protein with another part of the molecule in more of an aptamer fashion. They found they can upregulate expression by blocking the region of the lncRNA that binds to the protein using a short DNA oligonucleotide comprised of 50% LNA. Their initial target is the binding site for PRC2 on the lncRNA that appears to control transcription of the erythropoietin (EPO) gene. They demonstrated they were able to upregulate the production of EPO. Preliminary data showing a 25-fold upregulation of Erythrid Krüoppel-like Factor (EKLF; aka KLF-1) in mice was presented as well.
We expect that the story on lncRNA is just beginning and has the potential to far surpass siRNA because of what may soon be found to be a vast number of different functions in the cell.
RaNA Therapeutics was co-founded by Art Krieg, MD, who has more than 20 years of experience in immune stimulatory CpG oligonucleotide R&D in academia, and co-founded Coley Pharmaceutical Group (acquired by Pfizer) based on “CpG-ology,” as well as co-founded the first antisense journal, Oligonucleotides, and the Oligonucleotide Therapeutics Society. According to the RaNA Therapeutics website, “the capitalized “R”, “N”, and “A” in RaNA spell out RNA, the type of nucleic acid underlying our approach. The lowercase “a” represents activation. Together the letters stand for RNA activation, the very basis of our drug development approach.”
Getting Weird: Unnatural Base Pairs
Dr. Ichiro Hirao, RIKEN and President/CEO, TAGCyx Biotechnologies
As is usual for TIDES, there was very little “hard core” chemistry at this meeting. However, of the few chemistry talks, one of the more interesting was that given by Dr. Hirao of RIKEN. His research has been focused on the development of unnatural base pairs. He described the base pair that appears to be the winning combination, which consists of a purine analog (Ds) and a pyrimidine analog (Px) that can bind to each other with high affinity but not to the natural bases.
The authors demonstrated that these unnatural bases can also get incorporated by various polymerases with high fidelity. Misincorporation of the wrong base against these unnatural bases was only 0.005%, which is only slightly higher than the misincorporation rate of 0.002% for standard bases. The utility of the Ds base as a novel base for aptamer selection was described. Several random libraries that contained from one to three substitutions with the Ds base at specific locations were prepared. The oligos did not contain any Px bases, thus forming unusual structures that were not found in the natural sequence. This opened up the structural space for aptamer selection greatly. Using an ingenious method that substituted the Px base pair with a less specific base, Pa allows misincorporation of either dA or T at that site.
According to Wikipedia.org, RIKEN was founded in 1917 and has ~3000 scientists on seven campuses across Japan, the main one in Wako, just outside Tokyo. RIKEN is an Independent Administrative Institution whose formal name in Japanese is Rikagaku Kenkyūjo and in English is The Institute of Physical and Chemical Research. RIKEN conducts research in many areas of science ranging from basic research to practical applications. It is almost entirely funded by the Japanese government, and its annual budget is approximately $760 million.
JZ Comments: I wish to thank Rick Hogrefe for being a guest blogger and providing content! As always, I welcome reader comments!