
Super GMOs with a Super CRISPR/Cas9
May 2018
Last month in The CRISPR Journal researchers Wang Wei, et al. from Kansas State University demonstrated that improved wheat varieties can be generated using a multiplexed CRISPR-Cas9-based system.
Wheat is the leading grain produced worldwide based on acreage. As world population continues to expand and the environmental landscape becomes increasingly unpredictable, improving agronomic traits of this vital crop becomes increasingly important.
Astonishingly, despite the rise of genetically modified crops, there are still no commercial strains of genetically modified wheat. This is due to the difficulty in controlling heritability in the offspring plants. Wheat is allopolyploid (it inherits chromosomes from three parent plants) and it is estimated that traditional breeding strategies take as many as 7-10 years before target genes are fully integrated.
Multiplexed gene editing (MGE) strategies simultaneously edit multiple genes by introducing multiple gRNAs. Although they have previously been used in a variety of species, including wheat, MGE strategies have had limitations. With wheat, for example, researchers placed each gRNA under its own promoter in a single construct. However, due to the size of the individual promoter-gRNA units, the level of multiplexing was limited.
With this in mind, Wei and colleagues sought to overcome these obstacles utilizing a tRNA processing system to place multiple gRNAs under the control of one promoter. This allowed them to simultaneously introduce multiple gRNAs to edit the TaGW2, TaLpx-1, and TaMLO genes (see Figure 1). These genes affect wheat size, resistance to Fusarium graminearum, and resistance against powdery mildew, respectively.
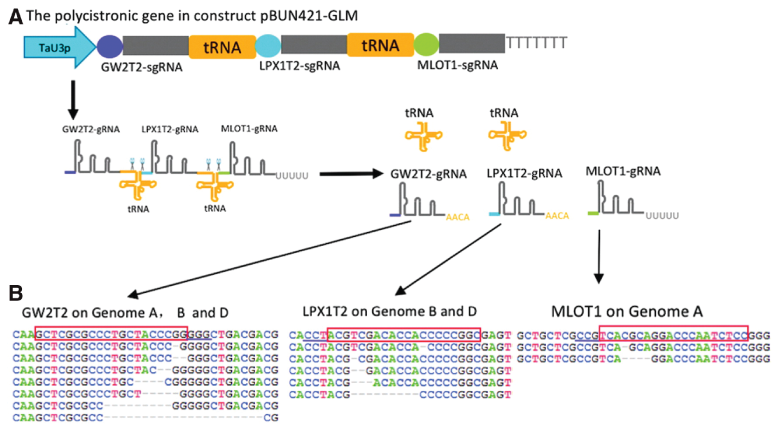
Additionally, the experiments were designed to test the feasibility of the selective editing of all three, two, or only one of the gene homologs in the allopolyploid wheat genome.
Analysis of the Cas9-induced mutations revealed that selective editing of the homologs was possible. Specifically, they found knockout mutations in all three homologous copies of TaGW2, and the TaGW2 and TaMLO genes were simultaneously present. While mutations at the TaLpx-1 gene were detected by NGS in multiple plants, they did not recover fixed heritable mutations at this site.
"However, it is possible that the ongoing CRISPR-Cas9 activity in the somatic cells can serve as a source of novel heritable mutations in the next generation of CRISPR-Cas9-expressing plants, thereby reducing the need for additional plant transformation experiments," note the authors.
Nonetheless, this is the first time that the knockout of all three copies of TaGW2 has been reported. This knockout resulted in a substantial increase in seed size and grain weight (see Figure 2), suggesting that the effects of editing multiple homologs may be additive.

It is clear that more research is needed to understand this strategy’s value in future wheat improvement, but the results to date look promising. This concept could vastly reduce the time for genetic integration currently held by traditional breeding strategies.
Relevant products: TriLink offers custom synthesis of gRNA oligos as well as several standard and custom variations of Cas9 mRNA. Please see our comprehensive list of stocked constructs here. If you need a unique Cas9 analog or incorporation of specific base modifications, please contact us to discuss our custom synthesis service.
Have a question? Visit Ask An Expert.
© 2018 TriLink BioTechnologies. | All Rights Reserved

