Safe and Effective Intracellular Transport to Respiratory Tract Epithelial Cells Without a Transfection Agent
- Simply Spray a Water Solution of Naked (Unformulated) mRNA
- Avoids Formulation in Lipid Nanoparticles
- Applicable to Neutralizing Antibodies and Therapeutic Proteins
Those of you who regularly read Zone blogs will recall the February 16, 2021 post on the recent demonstration of aerosol delivery of naked (i.e., unformulated) mRNA in water to epithelial cells in the female reproductive tract to generate neutralizing antibodies against HIV/AIDS.

As a follow-up to this remarkable achievement, which bypasses lipid nanoparticle LNP) carriers, this blog will highlight three additional reports on the successful transfection of epithelial cells simply using aerosols of aqueous naked mRNA. The achievement of these unassisted local deliveries of translatable naked mRNAs involving respiratory tract epithelia, two of them in mice and the other in horses, supports further development of this method as a treatment for respiratory diseases. Importantly, the naked mRNAs have chemically modified bases (modRNA) to evade the innate immune system and provide resistance to degradation by nucleases.
Introduction
Before providing short synopses of the aforementioned studies of aerosolized delivery of naked mRNA to the respiratory tract, the following information briefly outlines key features of the pulmonary barrier structure in the conducting and respiratory airways. According to a comprehensive review of delivery of aerosolized macromolecules for pulmonary pathologies by Osman et al., the pulmonary route is comprised of two structural parts with different physiological characteristics, as illustrated here: the conducing airways and the respiratory airways.
 The pulmonary barrier structure in the conducting and respiratory airways. Taken from Osman et al. and free to use. Open Access article distributed under the terms of the Creative Commons Attribution License 4.0.
The pulmonary barrier structure in the conducting and respiratory airways. Taken from Osman et al. and free to use. Open Access article distributed under the terms of the Creative Commons Attribution License 4.0.
The conducting or upper airways extend from the nose, pharynx, and trachea to the terminal bronchioles, and they are followed by the respiratory bronchioles, before ending at the alveolar sacs. The conducting part has columnar ciliated epithelium with mucous secreting cells that are responsible for clearing the airstream of exogenous particles, dust, and pathogens (see TriLink Research Update for more information) . The thickness of the epithelium is ~60 μm, with a thick mucus layer lined with a lung surfactant layer, and the surface area is ~2 m2. The main functions include transport of air to the gas-exchange area, as well as humidification, temperature adjustment, and filtering of the incoming air stream.
 Taken from commons.wikimedia.org and free to use.
Taken from commons.wikimedia.org and free to use.
The respiratory airways are distal to the terminal bronchioles (branching ~8-times) that end in alveolar sacs. There is a very thin (~0.1–0.2 μm) epithelium of alveolar cells and endothelium of blood capillaries. The relatively large surface area (~100–140 m2) facilitates gas exchange, displaying the highest permeability to water and macromolecules, which makes it a suitable target for drug delivery. The absorption of deposited particles occurs through either receptor-mediated transcytosis, paracellular passive transport via tight junctions, or various types of endocytosis, such as pinocytosis or receptor-mediated mechanisms depicted here.
Aerosol Delivery of Naked modRNA to Lungs of Mice for Therapy
The first investigation of a proof-of-concept for this novel approach for delivery of therapeutic proteins was published by Kormann et al. in 2011. Preliminary experiments employed a fluorescent reporter protein encoded in modRNA, wherein only 25% of uridine and cytidine were replaced with 2-thiouridine (2sU) and 5-methylcytidine (5mC) respectively, via the corresponding 5’-triphosphates obtained from TriLink, shown here. Intravenous administration to mice led to substantially decreased activation of the innate immune system while increasing the stability of the modRNA, thus allowing for prolonged high-level protein expression relative to the unmodified mRNA control.
Next, the researchers used mouse erythropoietin (mEpo) to explore whether similar modRNA has analogous effects in a physiologically functional model in vivo. Epo is a hormone produced by the peritubular capillary endothelial cells in the kidney and liver. It regulates red blood cell production and is used as a therapeutic in people who require dialysis.
Again, when compared to mice treated with unmodified mRNA, intramuscular administration of mice with the doubly substituted modRNA encoding mEpo significantly decreased activation of the immune system. 14 days thereafter, the mEpo levels were 4.8- and 4.4-fold higher compared to those in untreated mice or mice injected with unmodified mEpo mRNA, respectively. Concomitantly, the hematocrit (i.e., ratio of the volume of red blood cells to the total volume of blood) of mice injected with dual-modified mEpo modRNA increased to 64.2 ± 5.9% at day 28, which was significantly higher compared to the hematocrit of both mice treated with unmodified mEpo mRNA (54.2 ± 0.5%) and untreated mice (51.5 ± 1%).

Finally, Kormann et al. investigated the therapeutic potential of aerosol delivery of this mEpo modRNA in the lung by using a mouse model of congenital surfactant protein B (SP-B) deficiency, a rare, hereditary disease that leads to death soon after birth. It is refractory to surfactant replacement, and lung transplantation is the only therapeutic intervention currently available. The researchers used a conditional knockout mouse model for SP-B deficiency, in which the mouse SP-B cDNA was expressed under the control of exogenous doxycycline in SP-B−/− knockout mice.
Successful expression of SP-B in the lungs was confirmed by semiquantitative western blot analysis and immunostaining. SP-B modRNA–treated conditional SP-B−/− mice expressed 71.2 ± 8.3% of the amount of SP-B found in mice receiving doxycycline, whereas in mice treated with control modRNA, levels dropped to 1.8 ± 0.4%. Lung histology was close to normal in mice that were treated with SP-B modRNA, whereas lungs of mice that received control modRNA exhibited thickened alveolar walls, cellular infiltration, and interstitial edema after 4 days.
In summary, say Kormann et al., “these results demonstrate the…therapeutic efficacy of modified mRNA in a mouse model of a fatal human lung disease caused by the absence of SP-B.”
In a follow-up study reported in 2013, Kormann and collaborators used 10% 2-Thio-UTP and 5-Methyl-CTP to synthesize doubly modified modRNA encoding the regulatory T cell transcription factor FOXP3 for high-pressure intratracheal spray delivery in mice, in order to prevent allergic asthma in vivo. They showed that Foxp3 modRNA rebalanced pulmonary T helper cell responses, protecting from allergen-induced tissue inflammation, airway hyperresponsiveness, and goblet cell metaplasia in two asthma models. Notably, Foxp3 modRNA significantly increased FOXP3 expression when compared to unmodified mRNA or negative controls, achieving levels comparable to those of a conventional adeno-associated viral (AAV) vector.
Aerosol Delivery of Antibody-Encoded Naked modRNA to Mouse Lungs to Prevent Respiratory Syncytial Virus Infection
First, intratracheal aerosol delivery was employed to express the secreted form of the whole anti-RSV monoclonal antibody palivizumab (termed sPali) in mice, which reduced RSV infections by 91%.
Second, the well-characterized glycosylphosphatidylinositol (GPI) membrane anchor sequence was linked to the palivizumab heavy chain modRNA to afford anchored palivizumab (termed aPali) based on the hypothesis that cells transfected with aPali would retain the immunoglobulin on the epithelial surface, increasing its concentration in the lung and improving efficacy, as depicted here. This hypothesis was supported by the results of various in vitro experiments described in the original publication, along with extensive in vivo studies of the comparative efficacy of aPali modRNA administered in various ways vs. palivizumab and other controls.
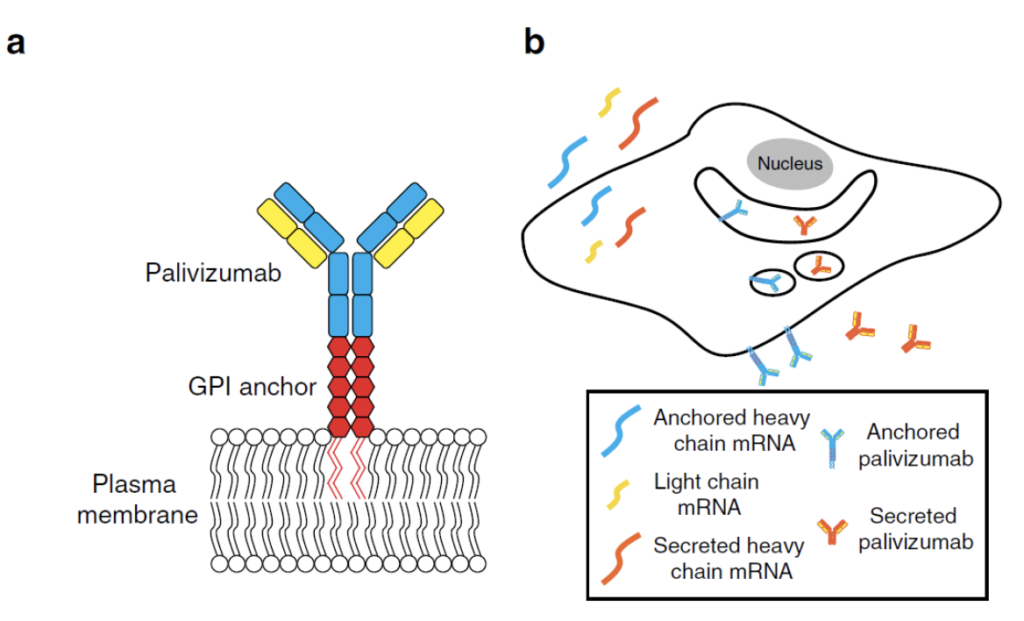
In terms of modRNA delivery comparisons, imaging data suggested to Tawari et al. that when delivered by aerosol to the lung, approximately half of the naked modRNA was free of the endosomal compartment, in contrast to typical lipid nanoparticle (LNP) delivery, in which only a small fraction of RNA is delivered to the cytosol. They added that “this clearly indicates that further research should focus on how droplet size, velocity, and buffers are facilitating cytosolic delivery.” Importantly, Tiwari et al. found that delivery of naked aPali modRNA was equally as effective as using Viromer RED, an agent from the Viromer polymers class comprised of a polycationic core of polyethyleneimine (PEI), which is highly substituted with hydrophobic and anionic side chains that bind nucleic acids to the core despite having an almost neutral surface charge.
Equally noteworthy, Tiwari et al. found that using the in vivo-jetPEI transfection agent exacerbated cytokine responses without significantly reducing viral titers, indicating the importance of delivery approach on outcomes and safety. In contrast, they found that delivery of modRNA diluted in water, without RSV infection-challenge, resulted in no significant inflammatory response. This result is critical, they say, especially in the lung, because inflammation can have severe consequences and a significant upregulation in cytokines could inhibit prophylactic protein expression.
Finally, Tiwari et al. used modRNAs to express an anchored or secreted high-affinity, anti-RSV, camelid antibody (termed RSV aVHH and sVHH, respectively) and demonstrated that RSV aVHH, but not RSV sVHH, significantly inhibits RSV 7 days post transfection. The researchers also showed that RSV aVHH is present in the lung for at least 28 days. Background on and applications of camelid antibodies (aka nanobodies) are described in a recent Zone blog at this link.
Aerosol Delivery of Naked modRNA to the Respiratory Tract of Horses
The most recent additional example of aerosol delivery of modRNA was provided in a January 2021 publication by Legere et al. in an in vivo study of respiratory tract epithelium in horses. They reasoned that the large surface area of the lungs allows for larger doses of modRNAs and much higher local concentrations of the transcribed protein, compared to traditional parenteral applications. More specifically, they aimed to transfect the airways of an equine model to potentially deliver immuno-therapeutic and immuno-prophylactic modRNAs. They demonstrated for the first time in vivo that transfection of the respiratory tract of a large animal can be done safely and effectively by aerosolizing naked modRNA in water.
To do this, Legere et al. used TriLink’s modRNA that encodes the enhanced form of green fluorescent protein (EGFP) and is optimized for transfection using 5-methoxyuridine (5moU) base modifications and CleanCap® (CleanCap® EGFP mRNA (5moU)).
More specifically, this modRNA is doubly U-modified with 75% 5moU and 25% Cyanine 5-U, the latter of which provides a commonly used dye (Cyanine 5 aka Cy5) to allow for visualization of the modRNA per se, distinct from its encoded EGFP protein following cytosolic translation.
When visualized with gel electrophoresis, the presence of both 5moU and Cy5-U modifications was shown to provide modRNA that was stable in PBS, saline, and water, as seen from this gel image.
The EGFP 5moU/Cy5 modRNA was formulated either as naked modRNA or with a commercially available Viromer for mRNA. These modRNA formulations were administered using an atomization device, either as an aerosol to cultured equine bronchial epithelial cells (EBECs) or as equine bronchial explants. When the EBECs and bronchial explants were submerged in cell media, successful translation of EGFP was only observed with the modRNA formulations that include Viromer. Failure to transfect with naked modRNA in vitro was attributed to enzymatic degradation of the naked modRNA by the culture media used for the EBECs or the explants (DMEM/F) (see gel image).
However, after temporarily removing the cell media, Legere et al. could also successfully transfect EBECs and bronchial explants with aerosolized naked modRNA. Moreover, when EGFP modRNA was entrapped in micron-sized droplets created by the atomizer, cells were transfected as efficiently and effectively with naked modRNA as they were with the modRNA prepared with Viromer.

Endoscopic images were collected to allow mapping back to the site of transfection on each side of the lung. Endoscopy was repeated at 8, 24, 48, and 96 h after aerosolization in both foals. At each time-point, they observed green fluorescence, indicating successful transfection of EGFP on the transfected side but not on the side aerosolized with water alone. Endoscopic examination demonstrated the absence of any gross changes to the aerosolized areas, and foals remained free of any clinical signs after each procedure and up to the end of the 14-day monitoring period post-transfection.
Legere et al. state that “this is the first report of in vivo evaluation of safety post-transfection without euthanasia and with several weeks of follow-up monitoring in a large animal model.” They also noted that the atomizer used in this study produces droplets of sizes between
30 to 100 μm in diameter and, while effective and safe, these modRNA droplets could only reach cells in close range of the tip of the aerosolizer. “Particle sizes < 5 μm in diameter must be generated to reach the lower airways via nebulization [and>
further studies to evaluate the effectiveness and efficiency of smaller particle sizes are warranted.”
Concluding Comments
The above-mentioned demonstrations of aerosol delivery of naked modRNAs and the results of their administration featured in the first part of this two-part blog collectively provide compelling support for additional preclinical studies of naked modRNA for certain therapies or as an alternative mode of vaccination. Interestingly, claims for unassisted (aka “gymnotic”) delivery of short-interfering RNA (siRNA) and phosphodiester-linked LNA to lung cells have been questioned.
While the utility of LNP-based delivery of unmodified RNAs or modRNAs has been firmly established, it is intriguing to speculate whether more extensive modifications of mRNA (i.e., “hypermodified” mRNA) might provide sufficient resistance to nucleases to allow broader clinical applications without special formulations.
What do you think?
Your comments are welcomed, as usual.
Please feel free to share this blog with your colleagues or on social media.







