Express more with premade and custom saRNAs
Drive the next wave of therapeutic breakthroughs with self-amplifying RNA (saRNA) constructs from TriLink BioTechnologies®. Compared to traditional mRNAs, saRNAs offer the advantages of lower dose requirements and durable, high protein expression, making them attractive for the development of innovative vaccines and gene therapies.
TriLink's ready-to-use catalog saRNAs ensure robust performance and serve as excellent controls for targeted delivery. For specific project needs, TriLink offers custom saRNAs constructs manufactured to your specifications, all backed by comprehensive quality control checks to help ensure consistent quality and reliable results.
Overview of saRNA
saRNA represents a major advancement in therapeutic development by enhancing gene expression through viral RNA's replicative mechanisms. This allows for high antigen or therapeutic protein levels from minimal RNA. With lower dosing, saRNA is particularly advantageous for vaccines and gene therapies, fostering strong immune responses and improving efficacy compared to traditional mRNA methods.
TriLink saRNA offerings to meet your project needs
saRNA synthesis
We offer custom self-amplifying RNA constructs to meet your specific experimental needs and project goals.
Purification options
Purification options for custom saRNA synthesis include silica membrane or oligo dT, ensuring quality and reliability.
Quality control
We conduct comprehensive quality control checks to ensure the integrity of every RNA product we deliver.
Ready-made saRNA controls
We provide catalog EGFP and FLuc saRNA constructs, with or without 5-methylcytidine (5mC) modification, for customer use in formulation and delivery optimization.
Custom saRNA synthesis capabilities
How to get a quote | mRNAbuilder® tool |
Target yield* |
|
NTP options |
|
saRNA vector |
|
5' cap options | |
RNA polymerase option | |
Purification options |
|
Formulation options |
|
Standard QC panel (included) |
|
Add-on saRNA characterization and analysis |
|
Release documentation** |
|
Total construct length |
|
Price |
|
TAT*** |
|
Delivery format |
|
* Target yield is provided as an estimate only, as the actual yield for an individual construct can be impacted by several factors, including sequence length and composition, capping type, and modifications.
**Analytical Reports (AR) are available with quicker turnaround times, released by manufacturing, and do not include a QC/QA review.
Certificates of Analyses (CoAs) include QC verification and release by QA and have a longer turnaround time than an Analytical Report.
***saRNA delivery time is dependent on the DNA template turnaround time and the complexity of the construct.
Ready to order your custom saRNA?
To order custom saRNA, use our mRNAbuilder tool and select "saRNA" from the type of mRNA to begin your build.
Ready-made catalog saRNAs
Accelerate your research with our formulation-ready catalog saRNAs for targeted delivery and optimization. Our saRNA vector used in our catalog is built on Venezuelan equine encephalitis virus (VEEV) vector with freedom to operate (FTO). The saRNA vector encodes for all the necessary components such as 5′ and 3′ UTRs, non-structural proteins (nsP1-4), a subgenomic promoter, and a 26-nt poly(A) tail.
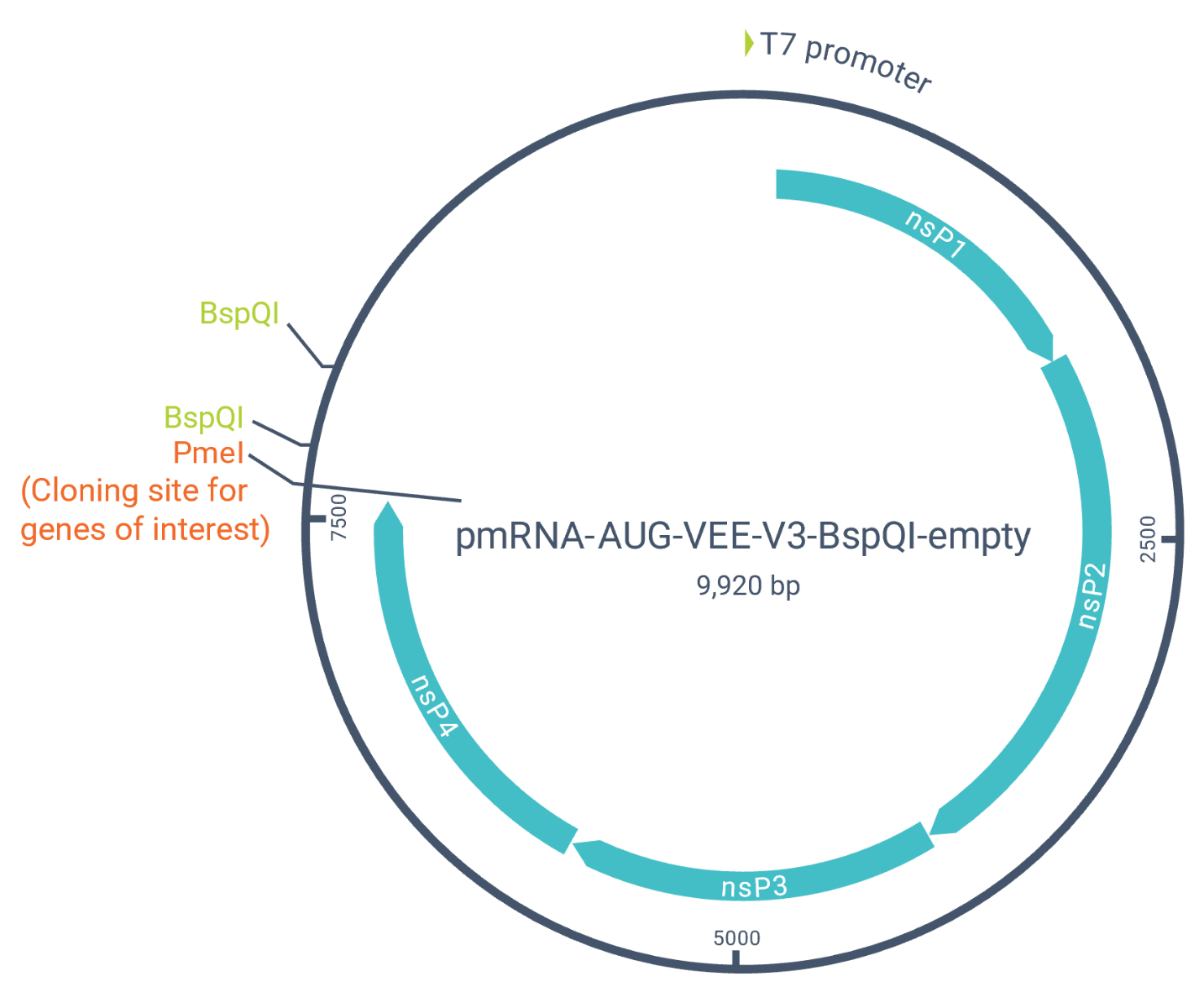
We manufacture our catalog saRNAs with the following features to help maximize your research benefits.
- Capped with CleanCap® Reagent AU which mimics the authentic 5′ alphavirus end while providing an optimal Cap-1 structure with >95% capping efficiency for efficient expression in mammalian cells.
- Synthesized using CleanScribe™ RNA Polymerase which reduces unwanted double-stranded RNA (dsRNA) by-products by up to 85%, for lower inflammatory responses and enhanced functionality.
- Manufactured using TriLink’s proprietary CleanScript® saRNA in vitro transcription (IVT) method which improves saRNA yield and integrity while also lowering dsRNA formation, for enhanced protein expression.
- Available with or without 5mC, the modification of which can reduce innate immune receptor activation and enhance translation of saRNAs.
Transition to GMP saRNA manufacturing
TriLink offers scalable and GMP-compliant solutions for saRNA manufacturing. With manufacturing processes developed specifically for saRNA, TriLink can produce material suitable for clinical and commercial applications. Schedule a consultation with one of our experts to discuss your project further.

Let’s discuss your saRNA project needs.
Complete the form below, and our support team will contact you shortly.
Frequently asked questions (FAQs) about saRNAs
While both saRNA and mRNA deliver instructions for protein production within cells, they have key structural and functional differences:
Length and structure: saRNA is generally longer than mRNA. This is because saRNA includes the coding sequence for alphavirus nonstructural proteins nsP1-4 to self-replicate within the cell. mRNA, on the other hand, does not contain such structure.
5′ cap: saRNAs are typically capped with AU to preserve the authentic alphavirus 5′ end whereas AG and GG caps are common for mRNAs.
Modified nucleotides: Both saRNA and mRNA often incorporate modified nucleotides to improve their functionality. saRNA frequently uses 5-methylcytidines (5mC or m5C), while N1-methylpseudouridine is a common modification in mRNA.
Protein expression: saRNA, due to its self-amplifying nature, generally results in sustained protein expression over a longer period. mRNA, while often providing a stronger initial burst of protein production, typically leads to shorter-lived expression as the RNA is not replicated.
We recommend storing the saRNAs at -40° C to -80 C. To minimize freeze-thaw cycles, aliquot the sample into single-use quantities on the first usage.
For saRNA capping, the use of an AU cap is recommended to preserve the authentic alphavirus 5′ end. The AU dinucleotide at the very terminus of the 5′ UTR is required for replication and may be needed for binding of the viral RNA dependent RNA polymerase complex (RdRp) (nsP4) to the 3′ end of the (-)sense strand for initiation of (+)strand synthesis. For details, please see Shirako et al. (2003).
CleanCap Reagent AU was specifically designed for saRNAs based on the genomes of (+)sense strand RNA viruses which start with a 5’ AU structure. Learn more about CleanCap Reagent AU in this tech note.
Modifications of saRNAs with 5-methylcytidines (5mC or m5C) have been reported to improve transfection efficiency, improve protein expression, and reduce inflammatory responses (Komori et al. (2023), Aboshi et al. (2024), McGee et al. (2024)).
We look for a single main band running to approximately the correct length to pass the gel result. Some factors such as modified NTPs can make a sample run slightly lower than the expected size. Sometimes, sequence-related factors such as highly repetitive or UTP-rich regions can result in additional bands. We take into account of all these factors to confirm that the saRNA was manufactured appropriately and the band is sequence specific before passing the results.
The fragment analyzer reports the percent of smear with a chromatogram. The value reported corresponds to the full-length integrity of an saRNA sample.


