CleanCap® mRNA capping technology
Accelerate therapeutic and vaccine development with an effective mRNA capping strategy
The 5’ cap structure is integral to the stability, expression, and immunogenicity of an mRNA product, but generating a synthetic cap can present manufacturing challenges and inefficiencies. The TriLink® patented CleanCap® technology offers:
- One-pot co-transcriptional capping strategy
- mRNA with an optimal Cap 1 structure
- Over 95% capping efficiency
Co-transcriptional capping with CleanCap® technology overcomes the limitations of legacy capping methods, helping you bring your mRNA vaccine or therapeutic to market faster.
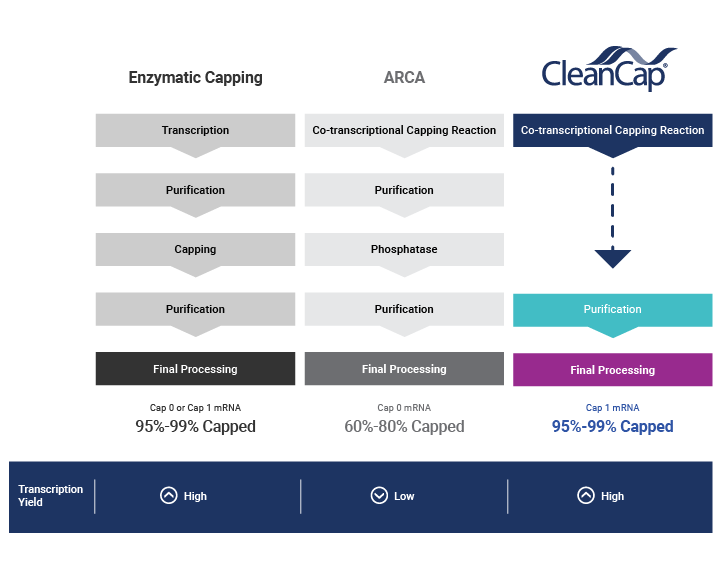
How does the CleanCap® technology work?
Learn about mRNA capping, co-transcriptional capping, and benefits of TriLink's patented CleanCap® capping technology.
Streamline mRNA manufacturing processes and reduce costs
As momentum grows in the mRNA therapeutic and vaccine pipeline, development timelines are critical in the race to reach the market. Leveraging TriLink's patented CleanCap® technology co-transcriptional capping solution, you can:
- Cut mRNA therapeutic production processes by nearly one week.
- Reduce overall manufacturing costs 20-40% lower than other capping methods.
By streamlining manufacturing, CleanCap® technology helps to ensure your mRNA program meets key development deadlines and cost-saving targets, increasing your likelihood of success in the path to the clinic.


White paper on the economics of mRNA capping strategies
Choose the right CleanCap® analog for your application
TriLink offers a robust portfolio of patented CleanCap® cap analogs for use in the discovery or GMP manufacturing of vaccines, cell therapies, gene replacement therapies, and more, ensuring an optimal mRNA capping strategy to help maximize mRNA potency and subsequent protein expression.
Our newest analog, CleanCap® M6, offers the most effective co-transcriptional method to date, increasing protein expression by over 30% when compared to the previous analogs.
Comparisons of CleanCap® analogs
| CleanCap ® M6 analog | CleanCap ® AG 3’ OMe analog | CleanCap ® AG analog | CleanCap ® AU analog | |
|---|---|---|---|---|
| Structure |  |  |  |  |
| Application | mRNA | mRNA | mRNA | Self-amplifying RNA |
| Cap modification(s) |
|
|
|
|
| Capping efficiency | > 95% | > 95% | > 95% | > 95% |
| Protein expression | Highest | Higher | High | High |
| Learn more | Learn more | Learn more | Learn more |
Related products and services

Modified NTPs
Our extensive modified nucleoside triphosphates, available in RUO- and GMP-grade, provide quality and flexibility in your RNA application.

N1-methylpseudouridine-5’-triphosphate
This popular modified NTP, available in both GMP- and RUO-grade, is for incorporation into mRNA using in vitro transcription.

mRNA synthesis and manufacturing services
Our manufacturing processes are fully customizable and scalable to support your needs for discovery, preclinical, and clinical projects.
Have questions about the CleanCap® technology? Discuss with one of our experts.
Complete the form below and our Business Development team will contact you shortly.


